Which statement defines specific heat capacity for given sample?
O the temperature of a given sample
1 %
the temperature that a given sample can withstand
O the quantity of heat that is required to raise the sample's temperature by 1°C (Kelvin)
O the quantity of heat that is required to raise 1 g of the sample by 1°C (Kelvin) at a constant pressure
Answers
The correct statement about specific heat capacity is " the quantity of heat that is required to raise 1 g of the sample by 1°C (Kelvin) at a constant pressure".
What is specific heat capacity ?The heat capacity of a given sample of a substance divided mostly by the mass of the sample, also known as mass heat capacity, is the specific heat capacity of that substance.
What is heat ?Heat is the energy transmitted from one body to the other as a result of a temperature differential. When two bodies of different temperatures collide, energy is transmitted.
Therefore, the quantity of heat that is required to raise 1 g of the sample by 1°C (Kelvin) at a constant pressure is correct statement.
To know more about heat and specific heat capacity.
https://brainly.com/question/13860901
#SPJ2
Answer: D. the quantity of heat that is required to 1 g of the sample 1°C (Kelvin) at a constant pressure
Explanation:
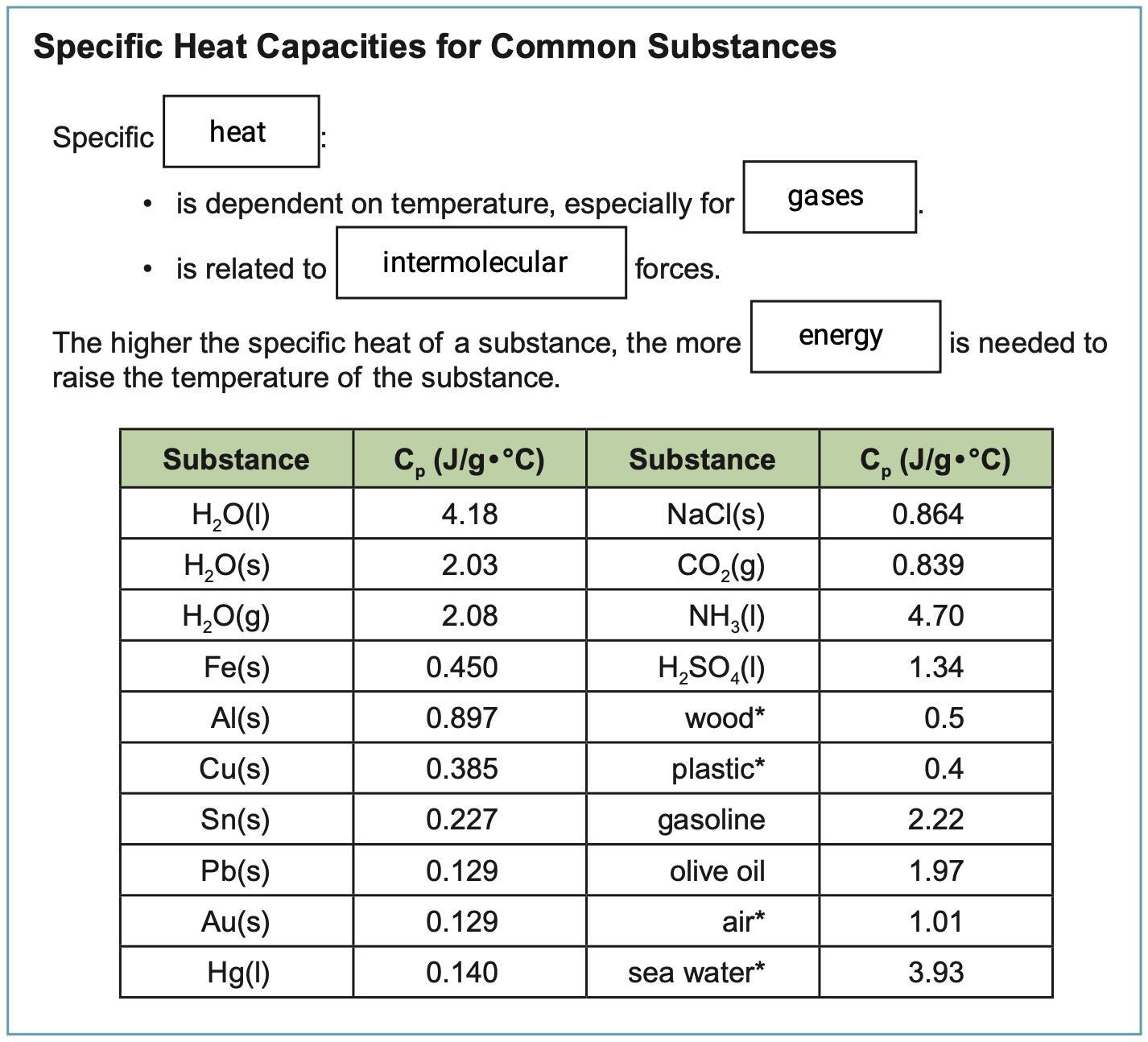
Specific heat capacity is defined as the quantity of heat required to raise the temperature of one gram of a substance by one degree Celsius (Kelvin) at a constant pressure.
It is dependent on temperature, especially for gases, and is related to intermolecular forces. The higher the specific heat of a substance, the more energy is needed to raise the temperature of the substance.
You can multiply this value by the mass of a given substance (g) and the change in temperature (degC) to find the amount of heat gained or lost (Joules).



Related Questions
Which is NOT a component of the kinetic theory of matter?
Answers
Answer:
1) all matter is made up of atoms and molecules 2) these tiny particles are always in motion; the higher the temp. the faster they move 3) at the same temp., heavier particles move more slowly then small particles
Explanation:
Question 14 (1 point)
A magnesium atom has 11 neutrons. What is the mass of this magnesium atom?
Answers
Answer:
Mass=23
Explanation:
Mass= Protons + neutrons
Atomic number is the number of protons in magnesium.
Atomic number =12...So there are 12 protons
12p + 11n = 23
A chemical reaction takes place in a closed system. The mass of the reactants before the reaction was 35 grams. What must the mass of the products of the reaction be, according to the law of conservation of mass?
Answers
A gas occupies a volume of 85.0 liters at a pressure of 2.24 atm and a temperature of 22.5 degrees celsius. How many moles of gas are in the container?
Answers
Answer:
n = 7.86 mol
Explanation:
This question can be solved using the ideal gas law of PV = nRT.
Temperature must be in K, so we will convert 22.5C to 295 K ( Kelvin = C + 273).
R is the ideal gas constant of 0.0821.
(2.24atm)(85.0L) = n(0.0821)(295K)
Isolate n to get:
n = (2.24atm)(85.0L)/(0.0821)(295K)
n = 7.86 mol
If you begin with 32.0 grams of oxygen and all of the hydrogen that you need, how many grams of water can you make?
Answers
Regarding water molecules, adhesion is best described as * the attraction water molecules have to other water molecules the attraction water molecules have to ionic substances the attraction water molecules have to polar substances the attraction water molecules have to other surfaces
Answers
Answer:
the attraction water molecules have to other surfaces
Explanation:
Adhesion is defined as the attractive forces between unlike substances, e.g water moving up a capillary tube.
Adhesion is the tendency of dissimilar particles or surfaces to cling to one another(Wikipedia).
So, what we mean by adhesion in this context, is the attraction water molecules to other surfaces.
6HCl(aq) + 2A1)
-
3H2(g) + 2AlCl3(aq)
1. How many moles of hydrogen are produced from 1.20 moles of aluminum?
Answers
Answer: 1.80 moles of [tex]H_2[/tex] will be produced 1.20 moles of aluminium.
Explanation:
To calculate the moles :
[tex]\text{Moles of solute}=\frac{\text{given mass}}{\text{Molar Mass}}[/tex]
The balanced chemical reaction is:
[tex]6HCl(aq)+2Al(s)\rightarrow 3H_2(g)+2AlCl_3(aq)[/tex]
According to stoichiometry :
2 moles of [tex]Al[/tex] produce = 3 moles of [tex]H_2[/tex]
Thus 1.20 moles of [tex]Al[/tex] will produce=[tex]\frac{3}{2}\times 1.20=1.80moles[/tex] of [tex]H_2[/tex]
Thus 1.80 moles of [tex]H_2[/tex] will be produced 1.20 moles of aluminium.
Which of the following is NOT an example of a chemical reaction?
A loaf of bread is sliced with a knife
A loaf of bread is baked in an oven
A loaf of bread is digested in the stomach
A loaf of bread molds in a pantry
Answers
Answer: D
Explanation:
because mold is a chemical reaction with with the air that's why the put a date cause the bread can only last for so long in till the chemical reaction.But D is is right because your body has to go though a chemical reaction in or to digest it and and break it down even more.So D would be the right answer cause that's where most of your chemical reaction happens when food is swallowed into the stoamch i hope i was able to explain it as much as i can
How will electron pairs behave towards one another according to VSEPR?
a. They will arrange themselves to be far apart from each other.
b. Electron pairs are not affected by other electron pairs.
c. They will be oriented close to each other.
d. The effect depends on the electronegativity of the atoms involved.
Answers
Ella is interested in combining substances to form a new material.
She read about some of the products chemists have discovered. She learned that sometimes synthetic materials are created accidentally. For instance, a chemist working on plastic gun sights synthesized a very sticky material accidentally. Which product had he accidentally made?
A. a coil spring toy
B. a heavy-duty adhesive glue
C. a new sweetener
D. a non-stick surface
please hurry
Answers
Sodium chloride is an ionic compound - it has a melting point of 800oC whereas chlorine is a gas has a melting point of -1200C. Explain the differences in melting points of these 2 in terms of their structure and bonding
Answers
Answer:
Our discussion up to now has centered on types of bonds that involve. This kind of bonding is called ionic bonding (as you are almost certainly already aware). NaCl is a solid at room temperature, with a very high melting point (801 °C), similar highly reactive metal – sodium (Na), and a pale green gas – chlorine (Cl2).
Explanation:
I hope this is what you needed. I also hope that you have a great day.
. Which of these choices would help explain why the Zebra mussel population was 0 in 1991, while the population of native Unionid mussels was over 3000 organisms per square meter?
a
The scientists had a hard time spotting the mussels that year.
b
The climate was too cold for the Zebra mussels to reproduce that year.
c
The Zebra mussels had not yet been introduced to the area.
d
The water was moving too fast for the Zebra mussels to survive that year.
2. Over the 18 years of data depicted here, which mussel species was generally more successful?
Unionid Mussels
Zebra Mussels
Answers
Answer:
C. The Zebra Mussels had not yet been introduced to the area.
2.
Unionid Mussels they had a headstart in 1990
Plants made the oxygen in the air animals use to breath by
Answers
Answer:
yeppppppppppppp
Answer:
yes
plants makes the oxygen by the
process of photosynthesis and
release to environment and animals
uses these oxygen to survive on this Earth
hope it helps
This graph shows how the potential energy of a reaction system changes
over time. Which statement is true for this system
A. The potential energy of the reactants is greater than the potential
energy of the products.
B. The height of the curve at point A represents the activation energy.
C. The height of the curve at point B represents the activation energy.
D. The potential energy of the products is greater than the potential
energy of the reactants.
Answers
Answer:
Answer is C since activation energy is needed in a reaction
Potential energy is the energy present in the reactions. The potential energy of the reactant in the graph is more than that of the product. Thus, option A is accurate.
What is potential energy?Potential energy is the energy of the reaction that results in the formation of products. The potential energy is reduced in the exothermic reactions as energy is released along with the products.
The bonds present in the reactants in the reaction mixture split in the presence of the activation energy to release energy and form the product.
Therefore, option A. the graph shows the greater potential energy of the reactant.
Learn more about potential energy here:
https://brainly.com/question/2292813
#SPJ2
*
What does the temperature measure?
A)Total Kinetic Energy of Matter
b) Average Potential Energy of Matter
C) Average Kinetic Energy of Matter
D)Total Potential Energy of Matter
Answers
Answer:
it would be C average kinetic energy
that is 137 words and thank ypou marked brainliest
Answers
Chemistry question just two
Answers
Answer:
14:Function 18:evaporation and distillation
Evaporation and distillation
How many grams are in
.093 liters of o2, gas at STP?
Answers
Answer:
0.13 grams of O2
Explanation:
Just did worksheet
Sulfur hexafluoride, SF6, is a very stable, nonflammable gas at room temperature. It
is used in many electronic products because it is a good electrical insulator.
Which statement describes the composition of sulfur hexafluoride?
Answers
The second answer choice is incorrect: while SF6 is indeed a compound that contains seven atoms, those atoms are not identical since one is a sulfur atom and six are fluoride atoms.
The third answer choice is incorrect: SF6 is not an element because it can be separated chemically into simpler substances that are elements. All the atoms comprising an element must have the same number of protons (the same atomic number); sulfur and fluorine have different atomic numbers. There is no single nucleus in SF6; the S and six F atoms each has their own nucleus.
The fourth answer choice is incorrect; SF6 contains no carbon atom, and so wouldn’t meet a central criterion for an organic compound. Moreover, the formula SF6 indicates that the sulfur atom is not bonded to any other atom apart from fluorine atoms, and there are no bonding electrons left on the S. There is no plausible reason to think that a molecule with such weak intermolecular attractions as SF6 would form long chains of S atoms. Indeed, in standard conditions, SF6 is a gas.
Sulfur hexafluoride (SF6) is a synthetic fluorinated compound with an extremely stable molecular structure.
What is sulfur hexafluoride?The first response option is the right one. A compound is a material made up of many atoms from various elements, such as SF6 (sulfur and fluorine).
The second option is wrong because, although SF6 does include seven atoms, those atoms are not all the same—six of them are fluoride atoms and one is a sulfur atom.
Because SF6 can be chemically divided into simpler chemicals that are elements, the third answer option is untrue. The six S and six F atoms each have their own nucleus, hence SF6 does not have a single nucleus.
Therefore, Sulfur hexafluoride (SF6) is a synthetic fluorinated compound with an extremely stable molecular structure.
To learn more about sulfur hexafluoride, refer to the link:
https://brainly.com/question/1478186
#SPJ5
Carbon C , reacts with excess sulfur dioxide , SO2 , to produce carbon disulfide, CS2 , and carbon dioxide, CO2 according to the following equation .
3C(s) + 2SO2(g) ⇉ CS2(I) + 2CO2(g)
How many grams of CS2 are produced when 6.00 moles of C reacts completely with excess amount of SO2 gas ?
Answers
Answer:
sorry sorry sorry sorry
If you have 4 moles of barium hydroxide, how many moles of oxygen atoms do you
have?
Answers
Answer:
8 moles of oxygen.
Explanation:
The chemical formula for barium hydroxide is Ba(OH)₂
This means one mole of barium hydroxide contains one mole of barium, two moles of hydrogen, and two moles of oxygen. If you have four moles of Ba(OH)₂ you will have four times the amount that is found in one mole.
4 moles of Ba(OH)₂ will have 8 moles of oxygen.
The number of moles of oxygen atoms we have in the compound is 8 moles .
The chemical equation for barium hydroxide is as follows:
Ba(OH)₂
According to the compound we have 1 atom of barium, 2 atoms of oxygen and 2 atoms of hydrogen.
Therefore, 4 moles of the compound can be represented as follows;
4 Ba(OH)₂
This means we have 4 atoms of barium, 8 atoms of oxygen and 8 atoms of hydrogen.
Therefore, we have 8 moles of oxygen atoms or 4 moles of oxygen molecules.
learn more: https://brainly.com/question/12228235?referrer=searchResults
What is 24.3 – 15.45 when accounting for sig fig rules?
Answers
Answer:
8.85 because im smart
3. What do you think is the primary reason why the Unionid mussel population declined whenever there is a dramatic increase in the Zebra mussel population?
Answers
When the Zebra arrived they ate all the food in the area leaving Unionid numbers to decline and die as they have no food to eat.
Doug used a magnet to move a .025kg ball. The magnet exerts a force of 5N, which causes the ball to begin moving. What is the acceleration of the ball when it begins to move?
Answers
Answer:
[tex]a=200\ m/s^2[/tex]
Explanation:
Given that,
The mass of a ball, m = 0.025kg
The force exerted by the magnet, F = 5 N
We need to find the acceleration of the ball when it begins to move. Let a be the acceleration. The net force of the ball is given by :
F = ma
Where
a is acceleration of the ball
[tex]a=\dfrac{F}{m}\\\\a=\dfrac{5}{0.025}\\\\a=200\ m/s^2[/tex]
so, the required acceleration of the ball is [tex]200\ m/s^2[/tex].
27. What element has this electron configuration: 1s2 2s22p6 352 3p6 452
3d10 4p4
Choices:
Sulfur
Selenium
Arsenic
Tellurium
Answers
Answer:
Selenium
Explanation:
One way to figure this out is to count the electrons and add them up.
2+2+6+2+6+2+10+4 = 34 electrons
A neutral atom has the same number of electrons and protons. Protons tell us the atomic number of an element. Element number 34 is Selenium.
Another way to figure it out is to look at just the highest "s" or "p" level of the electron configuration, and then see which element corresponds to that. This element has a 4s and a 4p, so I will look at 4p. The configuration is 4p4, so I will look at the 4th row of the periodic table (4p4) in the "p" block (4p4) and the 4th element in the "p" block (4p4). That's selenium :)
Nitric acid is a highly reactive acid with the chemical formula HNO3. It is a clear, colorless and odorless liquid at room temperature that is nonflammable. How many different elements make up one molecule of nitric acid? A. Three B. Four C. Five D. Two
Answers
Nitric acid, is a highly corrosive mineral acid. The pure compound is colorless, but older samples tend to acquire a yellow cast due to decomposition into oxides of nitrogen and water.
There are THREE different elements and a total of FIVE atoms in nitric acid.
The formula for nitric acid is HNO3. This means there is one atom of hydrogen, one atom of nitrogen, and three atoms of oxygen in one molecule of nitric acid.
So, Thus, your answer will be FIVE if you're counting the molecules but if you are counting the elements then it is THREE.
-Snooky
where do cells get the instructions on how to build proteins ? help
Answers
Answer:
Inside the DNA molecules
Answer:
Genes give instructions to proteins; protein structure determines traits.
Explanation:
Which of the following conditions creates the largest waves?
Question 3 options:
strong winds that blow in short spurts over a small distance
strong winds that blow over a great distance for a long time
strong winds that blow for a long time over small distances
Answers
Answer:
strong winds that blow in short spurts over a small distance
Explanation:
Generally, the most common factor that causes the largest waves in the ocean is winds. this is also called wind-driven waves of the ocean when the surface wind flows on the surface of the ocean, this disturbance creates the friction between surface wind and surface of the ocean which creates waves in the ocean
Answer:
B
Explanation:
k-12 test
Why are scientists worried that climate change will cause these toxic algae blooms to become more frequent?
Answers
Answer: Eutrophication is the enhancement of the growth of algae in the water body.
Explanation:
The scientists are worried for the climate change as if the climate changed to prolonged rainy then the frequent raining can remove toxic chemicals from the agricultural sites, landfills, industries, and from other locations and deposit them to the water body (river, lakes, ponds, and others). The deposition of the salts of nitrogen, phosphorus, and sulfur promotes the growth of algae in the water body. This leads to reduction in the concentration of oxygen in the water body. This is called eutrophication. The lack of oxygen can lead to mortality of aquatic animals.
How many cm are in 7 km?
Answers
Answer:
70000 cm in 7km
Explanation:
There are 1000 m and 100 cm in 1 m in 1 km, so to find how many cm are in 7 km you need to do 10000 x 7. You get 70000
Have a nice day!
Answer:
700000cm
Explanation:
1 km consists of 1000m and 1 m consists of 100 cm so 7×1000×100=700000cm