Answers
Answer:
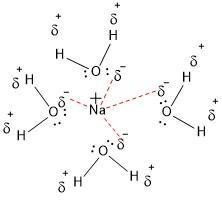
C. hydration number
Explanation:
When we dissolve an ionic compound (a charged species) the charges can interact with the water molecule. In the case of cations (positive charges) the negative dipole of water (generated in the oxygen) will interact with the positive charge at the same time the anions (negative charges) the positive dipole of water (generated in the hydrogen).
The amount of water molecules that can interact with a single ion (cation or anion) is called hydration number. In the example, we have a hydration number of "4" for the sodium cation.
I hope it helps!
Related Questions
Resources Use the exothermic and endothermic interactive to classify the solution process for each solute. Exothermic solution process Endothermic solution process
KOH CaCl, NaCT NaOH NaNO, NH NO,
Answers
Answer:
Exothermic interractive are the following: NaOH, KOH, CaCl₂
Endothermic interactive are the following: NaCl, NH₄NO₃, NaNO₃
Explanation:
NaOH, KOH, and CaCl2 are exothermic reactants. NaCl, NH4NO3, and NaNO3 are endothermic interacting substances.
Endothermic reactions: what are they?Chemical processes that can release or absorb energy are referred to as endothermic. In endothermic reactions, more energy is used when bonds in the reactants are broken than is released when new bonds are formed in the products.
How do endothermic processes take place?When the temperature of the an isolated system drops while the surroundings of the a non-isolated system warm up, this is known as an endothermic response. The heat of reaction is generally positive in endothermic processes (qrxn>0).
To know more about endothermic reactions visit:
https://brainly.com/question/23184814
#SPJ2
Identify the precipitation reaction in the set?
Answers
Answer:
The third reaction
(2NaOH + NiCL2 ---> 2NaCl + Ni(OH)2)
Explanation:
By definition, a precipitation reaction refers to the formation of an insoluble salt when two solutions containing soluble salts are combined.
(Source: lumenlearning)
From the 4 options, we can eliminate the first and second one immediately because there is no formation of an insoluble salt.
Then, the last one can also be eliminated because even though there is insoluble solid formed, but it is not a salt, and, the reactants are not solutions too. In fact, the last one is a displacement reaction. A more reactive metal displaces a less reactive metal to form an ion.
Since the third reaction matches the definition of precipitation reaction, this is the answer.
What is the rate constant of a reaction if rate = 1.5 (mol/L)/s, [A] is 1 M, [B] is
3 M, m = 2, and n = 1?
k=
rate
[A]" [B]"
A. 0.17
B. 13.5
C. 0.5
D. 4.5
Answers
[tex]\mathfrak{\huge{\pink{\underline{\underline{AnSwEr:-}}}}}[/tex]
Actually Welcome to the Concept of the Rate Constant.
Here, the "K" is the Rate Constant.
so the ANSWER IS C.) 0.5
The rate of constant is 0.5.
The answer is option C.
How do find the rate of constant?To determine the fee regulation from a desk, you have to mathematically calculate how differences in molar concentrations of reactants affect the response charge to parent out the order of every reactant. Then, plug in values of the response charge and reactant concentrations to discover the particular rate constant.
What's the rate of constant?The particular rate constant is the proportionality steady touching on the rate of the reaction to the concentrations of reactants. The fee regulation and the unique charge constant for any chemical response need to be decided experimentally. The price of the fee regular is temperature-established.
Learn more about the rate of constant here: brainly.com/question/8813467
#SPJ2
How many water molecules are in a block of ice containing 1.25 mol of water (H2O)
Answers
Answer:
Molecules = 7.5 × 10²³ molecules
Explanation:
Given:
Moles = 1.25 mol
Avogadro's No. = [tex]N_{A}[/tex] = 6.022 * 10²³
Required:
Molecules = ?
Formula:
Molecules = Moles × [tex]N_{A}[/tex]
Solution:
Molecules = 1.25 × 6.022 × 10²³
Molecules = 7.5 × 10²³ molecules
If 175mL of oxygen is produced at STP, how many grams of hydrogen peroxide, H2O2
were decomposed? At STP, 1 mole of gas occupies 22.4L. Be sure to balance first.
2 H202 > H202 + O2
what's the
Mass of H2O2
Answers
Answer:
0.53g
Explanation:
We'll begin by converting 175mL to L. This is illustrated below:
1000mL = 1L
Therefore 175mL = 175/1000 = 0.175L
Next, we shall calculate the number of mole of O2 that occupy 0.175L. This is illustrated below:
1 mole of O2 occupy 22.4L at stp.
Therefore, Xmol of O2 will occupy 0.175L i.e
Xmol of O2 = 0.175/22.4
Xmol of O2 = 7.81×10¯³ mole
Therefore, 7.81×10¯³ mole of O2 occupy 175mL.
Next, we shall determine the number of mole of H2O2 that decomposed to produce 7.81×10¯³ mole of O2. This is illustrated below:
2H2O2 —> 2H2O + O2
From the balanced equation above,
2 moles of H2O2 decomposed to produce 1 mole of O2.
Therefore, Xmol of H2O2 will decompose to produce 7.81×10¯³ mole of O2 i.e
Xmol of H2O2 = 2 x 7.81×10¯³
Xmol of H2O2 = 1.562×10¯² mole
Therefore, 1.562×10¯² mole of H2O2 decomposed in the reaction.
Finally, we shall convert 1.562×10¯² mole of H2O2 to grams. This is illustrated below:
Molar mass of H2O2 = (2x1) + (16x2) = 34g/mol
Mole of H2O2 = 1.562×10¯² mole
Mass of H2O2 =..?
Mole = mass /Molar mass
1.562×10¯² = mass /34
Cross multiply
Mass of H2O2 = 1.562×10¯² x 34
Mass of H2O2 = 0.53g
Therefore, 0.53g of Hydrogen peroxide, H2O2 were decomposition in the reaction.
Select the chemical equation that represents an acid base reaction??? Please help
Answers
Answer:
HCl + AgNO3 ------ AgCl + HNO3
Explanation:
HCl is an acid called hydrochloric acid, while AgNO3 is a base called Silver Nitrate.
Hope it helps.
Drag the description to the category
Answers
Answer:
ok
Explanation:
. Explain with examples following characteristics of chemical reactions:
a. Change of colour b. Evolution of gas c. Change of smell d. Change of state
Answers
Answer:
See explanation.
Explanation:
Hello,
a. In this case, the change of color is evident for instance when copper reacts with nitric acid to form hydrogen and copper (II) nitrate since copper orange-like and nitric acid is colorless, but copper (II) nitrate is green (dry) or blue (hydrated).
b. In this case, when we make react hydrochloric acid and magnesium, we notice a gas giving off while the magnesium chloride remains aqueous, due to the fact that magnesium displaces hydrogen which is given off as a gas.
c. In this case, we can consider an egg since when it is edible it has a tasty smell but when it decomposes to rotten egg, hydrogen sulfide is given off due to the action of specific bacteria, causing a change in smell to a quite stinky one.
d. In this case, a reaction by which a change of state is exhibited is for instance when aqueous lead (II) nitrate reacts with aqueous potassium iodide to yield potassium nitrate which remains aqueous whereas the lead (II) iodide precipitates out as a solid due to its tiny solubility as a yellow solid.
Best regards.
What volume (mL) of a concentrated solution of sodium hydroxide (6.00 M) must be diluted to 187 mL to make a 1.53 M solution of sodium hydroxide
Answers
Answer:
47.68 mL
Explanation:
In this case, we have a dilution problem. So, we have to start with the dilution equation:
[tex]C_1*V_1=C_2*V_2[/tex]
We have to remember that in a dilution procedure we go from a higher concentration to a lower one. With this in mind, We have to identify the concentration values:
[tex]C_1~=~6.00~M[/tex]
[tex]C_2~=~1.53~M[/tex]
The higher concentration is C1 and the lower concentration is C2. Now, we can identify the volume values:
[tex]V_1~=~X[/tex]
[tex]V_2~=~187~mL[/tex]
The V2 value has "mL" units, so V1 would have "mL" units also. Now, we can include all the values into the equation and solve for "V1", so:
[tex]6.00~M*V_1=1.53~M*187~mL[/tex]
[tex]V_1=\frac{1.53~M*187~mL}{6.00~M}=47.68~mL[/tex]
So, we have to take 47.68 mL of the 6 M and add 139.31 mL of water (187-47.68) to obtain a solution with a final concentration of 1.53 M.
I hope it helps!
The basic function of a carburetor of an automobile is to atomize the gasoline and mix it with air to promote rapid combustion. As an example, assume that 30 cm3 of gasoline is atomized into N spherical droplets, each with a radius of 2.0 × 10−5 m. What is the total surface area of these N spherical droplets? Answer: [A] m2.
Answers
Answer:
The total surface area of these N spherical droplets is 4.4929 m²
Explanation:
From the information given :
assuming that :
30 cm³ of gasoline is atomized into N spherical droplets &
each with a radius of 2.0 × 10−5 m
We are tasked to determine the total surface area of these N spherical droplets
We all known that:
[tex]1 \ cm^3 = 10 ^{-6} m^3[/tex]
Therefore
[tex]30 \ cm^3 = 30 * 10 ^{-6} m^3 = 3 *1 0^{-5} \ m^3[/tex]
For each droplet; there is a required volume which is = [tex]\dfrac{4}{3} \pi r ^3[/tex] since it assumes a sphere shape .
Thus;
replacing radius(r) with 2.0 × 10−5 m; we have:
[tex]= \dfrac{4}{3} \pi * (2.0 *10^{-5} m) ^3[/tex]
= [tex]3.35 * 10^{-14} \ m^3[/tex]
However; there are [tex]3*10^{-5} \ m^3[/tex] gasoline atomized into N spherical droplets with each with radius 2.0 × 10−5 m
For N ; we have ;
[tex]=\dfrac{3*10^{-5} \ m^3}{3.35 * 10^{-14} \ m^3/ droplet}[/tex]
= [tex]8.95*10^8 \ droplet s[/tex]
So; each droplet have a surface area = [tex]4 \pi r^2[/tex]
= [tex]4 \pi (2.0*10^{-5}m) ^2[/tex]
= [tex]5.02*10^{-9} \ m^2/droplets[/tex]
The surface area per droplet is equivalent to [tex]5.02*10^{-9} \ m^2/droplets[/tex]
Thus;
The total surface area of these N spherical droplets will be :
= [tex]8.95*10^8 \ droplet s * 5.02*10^{-9} \ m^2/ droplets[/tex]
= 4.4929 m²
The total surface area of these N spherical droplets is 4.4929 m²
Question 4
2 pts
A careless chemistry student performed a chemical reaction where his theoretical yield of
Magnesium oxide was 57.82 grams, but he actually produced 12.89 grams. What is his percent yield
for this experiment? (include the number with 4 significant figures but no units)
Answers
Answer:
22.29%
Explanation:
Percent yield = experimental yield / theoretical yield * 100
= 12.89 / 57.82 * 100 = 22.29%
What is the product(s) of the reaction below?
2Al(s) + Fe2O3(s) Al2O3(s) + 2Fe(s)
A. Solid aluminum oxide and solid iron
B. Solid aluminum
C. Saturated aluminum oxide and saturated iron
D. Iron(III) oxide and aluminum oxide
Answers
Answer:
I would put A
Explanation:
A new substance is produced as a result of a chemical reaction in which bonds between the molecules of the reactant and product are broken and new bonds are formed. Here the products are Al₂O₃ and Fe. The correct option is A.
Chemical reactions are interactions between two or more molecules that result in the production of new products. Products, as opposed to reactants, are compounds that result from an interaction between two other substances.
The reactants are on the left, while the products that are created are on the right. A one-headed or two-headed arrow connects the reactants and products.
Thus the correct option is A.
To know more about reaction, visit;
https://brainly.com/question/34137415
#SPJ4
g Which statement is incorrect regarding oxidation? Oxidation is a "gain" of electrons. Oxidation is the combination with O atoms. Oxidation is an increase in oxidation state. Oxidation is always accompanied by reduction. none of these
Answers
Answer:
The incorrect statement from the options is OXIDATION IS A "GAIN" OF ELECTRONS
Explanation:
Oxidation in a redox reaction is the loss of electrons. It is also the increase in the oxidation states of an atom or ion or atoms in a molecule. A redox reaction is a type of chemical reaction in which there is a transfer of electrons from an atom or ion to another resulting in a change in oxidation states of the substances involved. The reducing agent in the reaction is undergoes oxidation by losing electrons while the oxidating agent is reduced that is it gains electrons at the end of the reaction. The atom or ion from which electron is lost is said to be oxidized while the other atom or ion involved in the reaction is reduced.
Oxidation is also the combination with O atoms and it is always accompanied by reduction because oxidation forms a half of the whole redox reaction. A substance cannot be oxidized except it has reduced another substance by losing electrons to it.
At a certain temperature this reaction follows second-order kinetics with a rate constant of 0.00317sâ1: 2N2O5(g) â2N2O4(g) + O29(g) Suppose a vessel contains SO3 at a concentration of 1.44M . Calculate the concentration of SO3 in the vessel 0.240 seconds later. You may assume no other reaction is important.Round your answer to 2 significant digits.
Answers
Answer:
[A] = 1.438M = 1.4M (Two s.f)
Explanation:
Rate constant, k = 0.00317
Initial Concentration, [A]o = 1.44M
Final Concentration, [A] = ?
Time, t = 0.240 s
Since this is a second order reaction, the formula for this is given as;
1 / [A] = 1 / [A]o + kt
1 / [A] = 1 / 1.44 + (0.00317 * 0.240)
1 / [A] = 0.6944 + 0.0007608
1 / [A] = 0.6952
[A] = 1.438M = 1.4M (Two s.f)
As lead chemist for a pharmaceutical manufacturing company, you need to inform the purchasing office of a supply order for the next batch of cisplatin, PtCl2(NH3)2. If you intend to make a 500kg batch, how many kg chlorine gas do you need to order?
Answers
Answer:
mass of chlorine gas required is 118 kg.
Explanation:
Total mass of the drug (Cisplatin) required = 500 kg
For the drug PtCl2(NH3)2, we first find the molar mass of the compound.
The molar mass of the drug is the total of all the molar mass of the elements in the drug
molar mass of Pt (platinum) in the drug = 195.078 g/mol
molar mass of chlorine (Cl) in the drug = 2 x (35.453 g/mol) = 70.908 g/mol
molar mass of ammonia (NH3) in the drug = 2 x (17.031 g/mol) = 34.062 g/mol
Total molar mass of the drug = 195.078 g/mol + 70.908 g/mol + 34.062 g/mol = 300.048 g/mol
fractional composition of chlorine in the drug = 70.908/300.048 = 0.236
mass of chlorine required for 500 kg of the drug = 0.236 x 500 = 118 kg
A chemist observed an unknown Balmer Series decay through an emission of 410 nm. Using the experimental wavelength, determine the energy levels transition involved in the
emitted wavelength.
Answers
Answer:
Option D is correct.
n = 6 to n = 2
Explanation:
Like all waves emitted from the movement of electrons from one energy level to another, the wavelength (λ) is given by the equation involving Rydberg's constant
(1/λ) = Rₕ [(1/n₂²) - (1/n₁²)]
where Rₕ = 10973731.57 m⁻¹ = (1.0974 × 10⁷) m⁻¹
n₂ = principal quantum number corresponding to the final energy level of the electron = 2 (For Balmer Series)
n₁ = principal quantum number corresponding to the final energy level of the electron = ?
λ = 410 nm = (410 × 10⁻⁹) m
(1/λ) = (2.439 × 10⁶) m⁻¹
2.439 × 10⁶ = (1.0974 × 10⁷) [(1/2²) - (1/n₁²)]
0.25 - (1/n₁²) = (2.439 × 10⁶) ÷ (1.0974 × 10⁷) = 0.2222602562
(1/n₁²) = 0.25 - 0.2222602562 = 0.0277397438
n₁² = (1/0.0277397438) = 36.05
n₁ = 6
Hope this Helps!!!
A gas contained in a steel tank has a volume of 1.5 L at a temperature of 390 K. What will be the volume when the temperature changes to 1470C? Group of answer choices
Answers
Answer:
1.5 L
Explanation:
If the gas is contained in a steel tank, the volume will remain constant when the temperature changes.
The volume will be 1.5 L.
What would the cathode be in a nickel and copper electrolytic cell
Answers
Answer:
d
Explanation:
What is the molarity of a solution that is 7.00% by mass magnesium sulfate and has a density of 1.071 g/mL?
Answers
Answer:
0.623 M
Explanation:
Step 1: Given data
Percent by mass (%m/m): 7.00 %Density of the solution (ρ): 1.071 g/mLMolar mass of magnesium sulfate: 120.37 g/molStep 2: Calculate the percent by volume (%m/v)
We will use the following expression.
[tex]\%m/v = \%m/m \times \rho = 7.00\% \times 1.071g/mL = 7.50g\%mL[/tex]
Step 3: Calculate the molarity
7.50 g of magnesium sulfate are dissolved in 100 mL of the solution. The molarity is:
[tex]M = \frac{7.50g}{120.37g/mol \times 0.100L } = 0.623 M[/tex]
If an electromagnetic wave has a frequency of 4.5 x 10^18 Hz, what is its wavelength? The speed of light is 3 x 108 m/s.
Answers
Answer:
Wavelength, λ = 6.7 x 10^-11 m
Explanation:
Frequency and wavelength are inversely proportional to each other.
In this problem;
f = 4.5 x 10^18 Hz
wavelength, λ = ?
Speed of light, c = 3 x 108 m/s.
These variables are related by the following equation;
c = λ * f
Making λ subject of focus, we have;
λ = c / f
λ = 3 x 10^8 / 4.5 x 10^18
λ = 0.67 x 10^-10
λ = 6.7 x 10^-11 m
Two stereoisomers are obtained from the reaction of HBr with (S)-4-bromo-1-pentene. One of the stereoisomers is optically active, and the other is not. Draw the structure of the optically active stereoisomer.
Answers
Answer:
See explanation
Explanation:
In this case, we have an addition reaction. Additionally, this is a marknovnikov addition, therefore the "Br" atom would be added in the most substituted carbon (in this case carbon a). And we are going to have 2 enantiomers (2S,4S)-2,4-dibromopentane and (2R,4S)-2,4-dibromopentane. In the case of (2R,4S)-2,4-dibromopentane we will have a symmetry plane (a point in the molecule in which we can divide the molecule into two equal parts). When this happens we will have a mesocompound and we will not have optical activity.
See figure 1
I hope it helps!
A solid white substance A is heated strongly in the absence of air. It decomposes to form a new white substance B and a gas C. The gas has exactly the same properties as the product obtained when carbon is burned in an excess of oxygen. Based on these observations, can we determine whether solids A and B and the gas C are elements or compounds?
Answers
Answer:
A, B and C are compounds
Explanation:
First of all, I need to establish that when carbon is burnt in excess oxygen, carbon dioxide is obtained as shown by this equation; C(s) + O2(g) ----> CO2(g).
Looking at the presentation in the question, A was said to be heated strongly and it decomposed to B and C. Only a compound can decompose when heated. Elements can not decompose on heating. Secondly, compounds usually decompose to give the same compounds that combined to form them. Compounds hardly decompose into their constituent elements.
Again from the information provided, the compound A is a white solid. This is likely to be CaCO3. It decomposes to give another white solid. This may be CaO and the gas was identified as CO2.
Hence;
CaCO3(s)--------> CaO(s) + CO2(g)
Write the empirical formula
Answers
Answer:
[tex]Pb(CO_{3})_{2} \\Pb(NO_{3})_{4} \\FeCO_{3}\\Fe(NO_{3})_{2}[/tex]
Explanation:
[tex]Pb^{4+}(CO_{3}^{2-})_{2} --->Pb(CO_{3})_{2} \\Pb^{4+} (NO_{3}^{-})_{4} --->Pb(NO_{3})_{4} \\Fe^{2+} CO_{3}^{2-} --->FeCO_{3}\\Fe^{2+} (NO_{3}^{-})_{2}--->Fe(NO_{3})_{2}[/tex]
At a temperature of 393 K, the pressure of a sample of nitrogen is 1.07 atm. What will the pressure be at a temperature of 478 K? (Assume constant volume)
Answers
Answer:
1.30atm
Explanation:
P1/T1 = P2/T2
1.07/393 = P2/478
Answer: the first one is correct
Explanation:
jhkfjfjgjgjjggj
A 3.00-g sample of an alloy (containing only Pb and Sn) was dissolved in nitric acid (HNO3). Sulfuric acid was added to this solution, which precipitated 2.93 g of PbSO4. Assuming that all of the lead was precipitated, what is the percentage of Sn in the sample? (molar mass of PbSO4 = 303.3 g/mol)
Answers
Answer:
33.3% of Sn in the sample
Explanation:
The addition of SO₄⁻ ions produce the selective precipitation of Pb²⁺ to produce PbSO₄.
Moles of PbSO₄ (molar mass 303.26g/mol) in 2.93g are:
2.93g ₓ (1mol / 303.26) = 9.66x10⁻³ moles PbSO₄ = Moles Pb²⁺.
As molar mass of Pb is 207.2g/mol, mass in 9.66x10⁻³ moles of Pb²⁺ is:
9.66x10⁻³ moles of Pb²⁺ ₓ (207.2g / mol) = 2.00g of Pb²⁺
As mass of the sample is 3.00g, mass of Sn²⁺ is 3.00g - 2.00g = 1.00g
And the percentage of Sn in the sample is:
1.00g / 3.00g ₓ 100 =
33.3% of Sn in the sampleA sample of magnesium ribbon is ignited in a crucible to form magnesium oxide. Determine the empirical formula of magnesium oxide from the following data:
mass of crucible and cover + magnesium metal
33.741 g
mass of crucible and cover
33.500 g
mass of crucible and cover + magnesium oxide
33.899 g
Answers
Answer:
MgO
Explanation:
The following data were obtained from the question:
mass of crucible and cover + magnesium metal = 33.741 g
mass of crucible and cover = 33.5 g
mass of crucible and cover + magnesium oxide = 33.899 g
Next, we shall determine the mass of magnesium metal. This can be obtained as follow:
mass of crucible and cover + magnesium metal = 33.741 g
mass of crucible and cover = 33.5 g
Mass of magnesium metal =..?
Mass of magnesium metal = (mass of crucible and cover + magnesium metal) – (mass of crucible and cover)
Mass of magnesium metal = 33.741 – 33.5
Mass of magnesium metal = 0.241g
Next, we shall determine the mass of magnesium oxide. This can be obtained as follow:
mass of crucible and cover + magnesium oxide = 33.899 g
mass of crucible and cover = 33.5 g
Mass of magnesium oxide =?
Mass of magnesium oxide = (mass of crucible and cover + magnesium oxide) – (mass of crucible and cover)
Mass of magnesium oxide = 33.899 –. 33.5
Mass of magnesium oxide = 0.399g
Next, we shall determine the mass of oxygen. This can be obtained as follow:
Mass of magnesium oxide = 0.399g
Mass of magnesium metal = 0.241g
Mass of oxygen =..?
Mass of oxygen = (Mass of magnesium oxide) – (Mass of magnesium metal)
Mass of oxygen = 0.399 – 0241
Mass of oxygen = 0.158g
Now, we can obtain the empirical formula for the magnesium oxide as follow:
Mg = 0.241g
O = 0.158g
Divide by their molar mass
Mg = 0.241 / 24 = 0.01
O = 0.158 / 16 = 0.0099
Divide by the smallest
Mg = 0.01 / 0.0099 = 1
O = 0.0099 / 0.0099 = 1
Therefore, the empirical formula for the magnesium oxide is MgO
A compound consisting of atoms of small atomic mass is more likely to require what
Answers
Answer:
a lower temperature to liquefy
Explanation:
What energy transfer happens when wood is burning?
Answers
Answer:
Mechanical to Heat
explanation:
The wood itself can make mechanical energy but when it's on fire it makes heat energy
Answer: Chemical to heat and light
Explanation: The energy transforms from chemical energy to heat and light energy. Because when the candle burns a chemical reaction occurs and produces heat and light.
The mathematics of combining quantum theory with wave motion of atomic particles is known as _____.
Answers
Combining quantum theory with wave motion of atomic particles is: Wave Mechanics
Morphine, C 17H 19NO 3, is often used to control severe post-operative pain. What is the pH of the solution made by dissolving 25.0 mg of morphine in 100. mL of water? (For morphine, K b = 1.62 × 10 –6.)
Answers
Answer:
pH = 9.58
Explanation:
First of all, we need to determine the molarity of the solution.
We determine the molar mass of morphine:
12g/m . 17 + 1 g/m . 19 + 14 g/m + 16 g/m . 3 = 285.34 g/m
molar mass g/m, is the same as mg/mm
25 mg . 1 mmol / 285.34 mg = 0.0876 mmoles / 100 mL = 8.76×10⁻⁴ M
In diltuted solution, we must consider water.
Mass balance for morphine = [Morphine] + [Protonated Morphine]
8.76×10⁻⁴ M = [Morphine] + [Protonated Morphine]
As Kb is too small, I can skipped, the [Protonated Morphine]
8.76×10⁻⁴ M = [Morphine]
In the charge balance I will have:
[OH⁻] = [H⁺ morphine] + [H⁺]
Let's go to the Kb expression
Morphine + H₂O ⇄ MorphineH⁺ + OH⁻ Kb
Kb = [MorphineH⁺] [OH⁻] / [Morphine]
Kb = [MorphineH⁺] [OH⁻] / 8.76×10⁻⁴ M
So now, we need to clear [MorphineH⁺] to replace it in the charge balance
Kb . 8.76×10⁻⁴ M / [OH⁻] = [MorphineH⁺]
Now, the only unknown value is the [OH⁻]
[OH⁻] = Kb . 8.76×10⁻⁴ M / [OH⁻] + Kw/[OH⁻]
Remember that Kw = [H⁺] . [OH⁻]
[H⁺] = Kw/[OH⁻]
[OH⁻]² = 1.62×10⁻⁶ . 8.76×10⁻⁴ + 1×10⁻¹⁴
[OH⁻] = √(1.62×10⁻⁶ . 8.76×10⁻⁴ + 1×10⁻¹⁴)
[OH⁻] = 3.76×10⁻⁵ → - log [OH⁻] = pOH = 4.42
pH = 14 - pOH → 14 - 4.42 = 9.58