Answers
Answer:
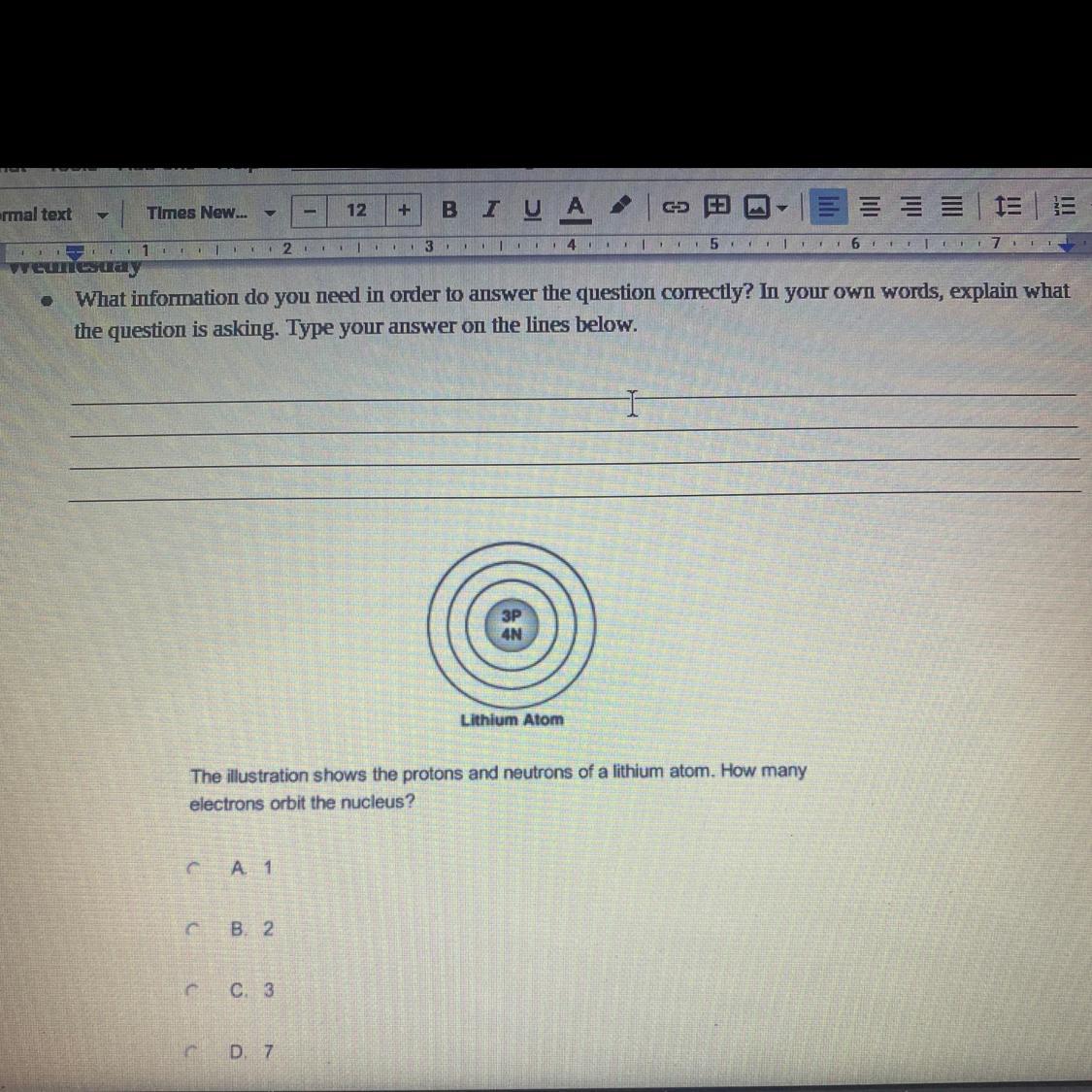
I believe the answer is C (3)
Explanation:
In an atom usually electrons number and protons number is equal.
Here lithium atom has three protons
So it has three electrons، too.
Related Questions
What is the purpose of the title of a line graph
Answers
Answer:
It tells about the data for each line graph is plotted.
Explanation:
Answer:
It can describe what the line graph is measuring and how it is measuring it
If the density of a substance is 2.18 g/mL, how would you find the density given that the mass is 0.987 kg and the volume is 4.52 x 102 mL?
Answers
Answer:
Explanation:
Convert mass in gms .. 987 gms
Density = mass/ volume
= 987/4.52 x102
= 2.18 g/mL
Which describes the elements in a given group of the periodic table?
They decrease in atomic mass as you go down the column.
They increase sequentially in atomic number from top to bottom.
They are in the same family, with similar characteristics.
Their properties change according to a pattern as you go down the column.
Answers
Answer:
They are in the same family, with similar characteristics.
Explanation:
The elements in a given group of the periodic table are in the same family and with similar characteristics.
Vertical columns on the periodic table are referred to as a group or family. Elements having the same number of electrons in the outermost shell of their atoms are placed one another in vertical columns. Each resulting vertical column is a group or family. Elements in the same group exhibit similar chemical properties because of the same number of electrons in their atoms' valence shells.Calculate the volume 3.00 moles of a gas will occupy at 24.0 °C and 762.4 mm Hg.
Answers
Answer:
Use formula of PV = nRT
Change formula to solve for Volume = nRT/P
R is a constant 0.08206
Convert C to Kelvin which is just +273
And turn mmHg into atm which is around 1 atm
Plug in and solve
At every depth, soil composition remains the same.
O
True
O False
Answers
Answer:
false
Explanation:
Hope the answer was usefull for us
Answer:
false. there is the surface soil, which has a lot of partly decayed stuff, topsoil, which has minerals and organic material, subsoil, which is made from sand, silt, and clay that hasn't been broken down, and parent material, which is the place where not much living things live in, except for gigantic tree roots. then there is the bedrock, which is; you guessed it:rock entirely.
Explanation:
how do you write out 4,5-dimethyl-4-octene
Answers
you can see the answer at the pic
Neturally an atom is neutral in charge why
Answers
Answer
Explanation:
an atom is usually define as a perfect balance of electrons and protons
Do you guys have viruses wit this website
Answers
what is the definition of chemical formula?
Answers
Answer:
A chemical formula is a way of presenting information about the chemical proportions of atoms that constitute a particular chemical compound or molecule, using chemical element symbols, numbers, and sometimes also other symbols, such as parentheses, dashes, brackets, commas and plus and minus signs. These are limited to a single typographic line of symbols, which may include subscripts and superscripts. A chemical formula is not a chemical name, and it contains no words. Although a chemical formula may imply certain simple chemical structures, it is not the same as a full chemical structural formula. Chemical formulae can fully specify the structure of only the simplest of molecules and chemical substances, and are generally more limited in power than are chemical names and structural formulae.
Explanation:
Have A Wonderful Day !!
Using the Periodic Table predict which elements will have similar chemical properties or reactivity.
Group of answer choices
rubidium, yttrium, and zirconium
nitrogen, sulfur, and bromine
magnesium, strontium, and barium
cadmium. calcium, and carbon
Answers
Answer:
magnesium. stronsium. barium. they are have similar group in IIA
To compare how different types of soil absorbed water, Christian set up an experiment. He poked equal numbers and sizes of holes into the bottoms of three plastic cups. He filled one cup with sand, one with potting soil, and left one empty. He placed each cup in a stand over a beaker. Christian poured 400 mL (milliliters) of water into each of the cups. He then measured the amount of water collected in the beakers beneath each cup at 1 minute intervals for 3 minutes. What is the control in this experiment? Group of answer choices
Answers
Answer:
The empty cup
Explanation:
Got it right on the test
Given: (52.6 cm)(1.214 cm)
What is the product expressed to the correct number of significant figures?
1.
64 cm2
2.
63.9 cm2
3.
63.86 cm2
4.
63.8564 cm2
Answers
Answer:
2.
63.9 cm2
Explanation:
All non-zero digits are consider significant figures like 1, 2, 3, 4, 5, 6, 7, 8, 9.
Leading zeros are not consider as a significant figures. e.g. 0.06 in this number only one significant figure present which is 6.
Zero between the non zero digits are consider significant like 108 consist of three significant figures.
The zeros at the right side e.g 240000 are also significant. There are six significant figures are present.
When we add or subtract the values the number of significant figures after decimal in result must be equal to the given measurement having less number of decimal places. For example,
The difference between 7.69 and 4.0.
7.69 - 4.0 = 3.7
When we multiply or divide the values the number of significant figures must be equal to the less number of significant figures in given value.
For example in given q:
52.6 cm × 1.214 cm = 63.9 cm²
Whas the the ionic radius of strontium
Answers
Answer: 255 pm
Explanation: Hope this helps!
Is a backpack a good resource
for a rescue team?
Answers
How does an object's mechanical energy change as its speed (velocity) increases?
Answers
Answer:
The mechanical energy of the system increases, provided there is no loss of energy due to friction. The energy would transform to kinetic energy when the speed is increasing. The mechanical energy of the system remains constant provided there is no loss of energy due to friction.
Explanation:
32 N of Force. 37 N force. How strong is the net force and in which direction?
Answers
Explanation:
They are working against each other. That means you subtract.
32 - 37 = -5
You are moving 5 units to the left because 37 is negative which means it is moving left. The key word is left. In physics, you would get the same answer if you did it this way.
37 - 32 = 5
The 5 moves in the same direction as the 37
Lime stone is a sedimentary rock that contains calcium this type of rock typically forms in warm shallow marine waters. Which process is involved in distributing calcium before it becomes part of the sedimentary rock?
A) tectonic movement
B) weathering
C) a biological process
D) volcanic activity
Answers
Answer:
the answer is B!!!
Explanation:
During the Metric Olympics yesterday, Molly's long jump was 118.3 cm. Christy accidentally recorded her results in inches. She jumped 51.18 inches. Can you help... Who won the long jump? Christy won by about 30 cm Christy won by about 30 cm Molly won by about 25 cm Molly won by about 25 cm Christy won by about 12 cm Christy won by about 12 cm Molly won by about 10 cm
Answers
Answer: Christy won Molly by 12cm
Explanation:
Ist step
Molly's ;ong jump was 118.3cm
Christy long jump was 51.18inches
2nd Step
we change christy score to cm so we can compare
1 inch = 2.54cm.
therefore 51.18 inches = 51.18 x 2.54= 129.9972cm
Christy won molly by 129.9971cm-118.3cm=11.69 rounded to 12cm
Christy won Molly by 12cm
What is an ionic bond?
Answers
Answer:
Ionic bonding is the complete transfer of valence electron(s) between atoms. It is a type of chemical bond that generates two oppositely charged ions. In ionic bonds, the metal loses electrons to become a positively charged cation, whereas the nonmetal accepts those electrons to become a negatively charged anion
Explanation:
pleaseee mark brainliest <3!
What causes the air above a pot of boiling water to become warm?
The air transfers thermal energy to the water vapor.
The water vapor transfers thermal energy to the air.
The particles in the air lose kinetic energy.
The particles in the water vapor gain kinetic energy.
Answers
Answer: The water vapor transfers thermal energy to the air.
Explanation:
In reality, energy conversion from burning fuel is never 100% efficient. Significant loss of energy due to heating occurs. If the generator were only 20% efficient (which is more realistic), how much energy in joules would be converted to electrical energy and how much would be lost to heat? (2 points)
Answers
Answer:
Electrical energy = 130000000 J and Heat energy = 520000000 J
Explanation:
Multiply the amount of joules from the last question (650000000) by .20 and .80. (Which are the percentages)
choose the letter that best describes the behavior of a substance during a phase change:
/A/ neither absorbs or releases energy
/B/ always absorbs energy
/C/ always releases energy
/D/ either absorbs or releases energy
Answers
Answer:
/D/ either absorbs or releases energy
Explanation:
During a phase change energy is either absorbed or released.
A phase change is a reversible process which occurs when a substance changes is its state from one form to another.
Phase changes are used to delineate physical changes.
A phase diagram provides a graphic representation of the change. The diagram can be 2 dimensional or 3 dimensional.Noble gas configuration
Answers
Answer:
Rubidium= [Kr] 5s^1
Calcium= [Ar] 4s^2
Aluminium= [Ne] 3s^2 3p^1
Explanation:
A noble gas configuration begins with the elemental symbol of the last noble gas prior to the atom. The symbol is then followed by the remaining electrons.
Hope this helped! good luck :)
What is the decomposition of 2LiCIO3
Answers
2LiClO3 —> 2LiCl + 3O2
A young girl has blue eyes.
Which statement explains how the young girl inherited her blue eye color?
A. The girl received proteins from her mother and father that coded for blue eye color.
B. The girl received blue pigments from her mother and father that formed her blue eye color.
O c The girl received cells from her mother and father that blended together to form her blue eye color.
D. The girl received chromosomes from her mother and father containing DNA that coded for blue eye color.
Answers
Answer:
D
Explanation:
( I hope that this helps )
Answer:
The answer is d
Explanation:
2. Calculate the density of a rock that has a mass of 21.58 grams and causes the water in a
graduated cylinder to rise from 20.0yl to 25.4 ml.
Answers
Answer:
4.00 g/mL
Explanation:
Density is mass divided by volume (g/mL).
Mass of rock = 21.58
Volume of rock = 25.4 - 20.0 = 5.4mL
Density of rock = 21.58g / 5.4mL = 3.99629 g/mL
Round to the lowest number of significant figures which is three = 4.00 g/mL
Explain three ways the periodic table is organized. Include in your essay the
definition of two listed trends and the patterns observed for each of your selected
trends on the periodic table
Answers
Answer:
The periodic table is organized in non-metals metals and metalloids, in in terms of trends and patterns you may want to talk about the the density of the elements or the mass of per unit of measurement of whatever you're going to use sorry that could be much help but I hope it helps.
medicine some chemical components (chemical formula)
Answers
Answer:
A chemical formula is a way of presenting information about the chemical proportions of atoms that constitute a particular chemical compound or molecule, using chemical element symbols, numbers, and sometimes also other symbols, such as parentheses, dashes, brackets, commas and plus (+) and minus (−) signs.
Compounds used as medicines are most often organic compounds, which are often divided into the broad classes of small organic molecules (e.g., atorvastatin, fluticasone, clopidogrel) and "biologics" (infliximab, erythropoietin, insulin glargine), the latter of which are most often medicinal preparations of proteins
Explanation:
What is the mass of 0.250 mol of chlorotrifluoromethane, CCIF3?
A. 26.1 g
B. 104 g
C. 4.78 x 10-3 g
D. 52.2g
Answers
Answer:
26.1g
Explanation:
Cclf3
=35.5
F=54/101.5
Mass=mol*mm
=0.25*1015
=26.1g
Please i need to pass this!
Please try
Answers
It is incorrect because it should have a total of eight electrons with two electrons between each H and O
Further explanationLewis structure shows the form of bonds that occur in the valence electrons of an element or compound
This bonding usually occurs in covalent bonds
Valence electrons are electrons used in a chemical bond
The main group elements usually have valence electron configurations in the ns and np subshells, While the transition elements in the subshells (n-1) d and ns.
Element O has 6 valence electrons
Element H has 1 valence electron
To achieve stability, the element O needs 2 more electrons which are obtained from 2 electrons from the H element, while the H element needs 1 electron to be stable
So that a covalent bond is formed