Answers
Answer:
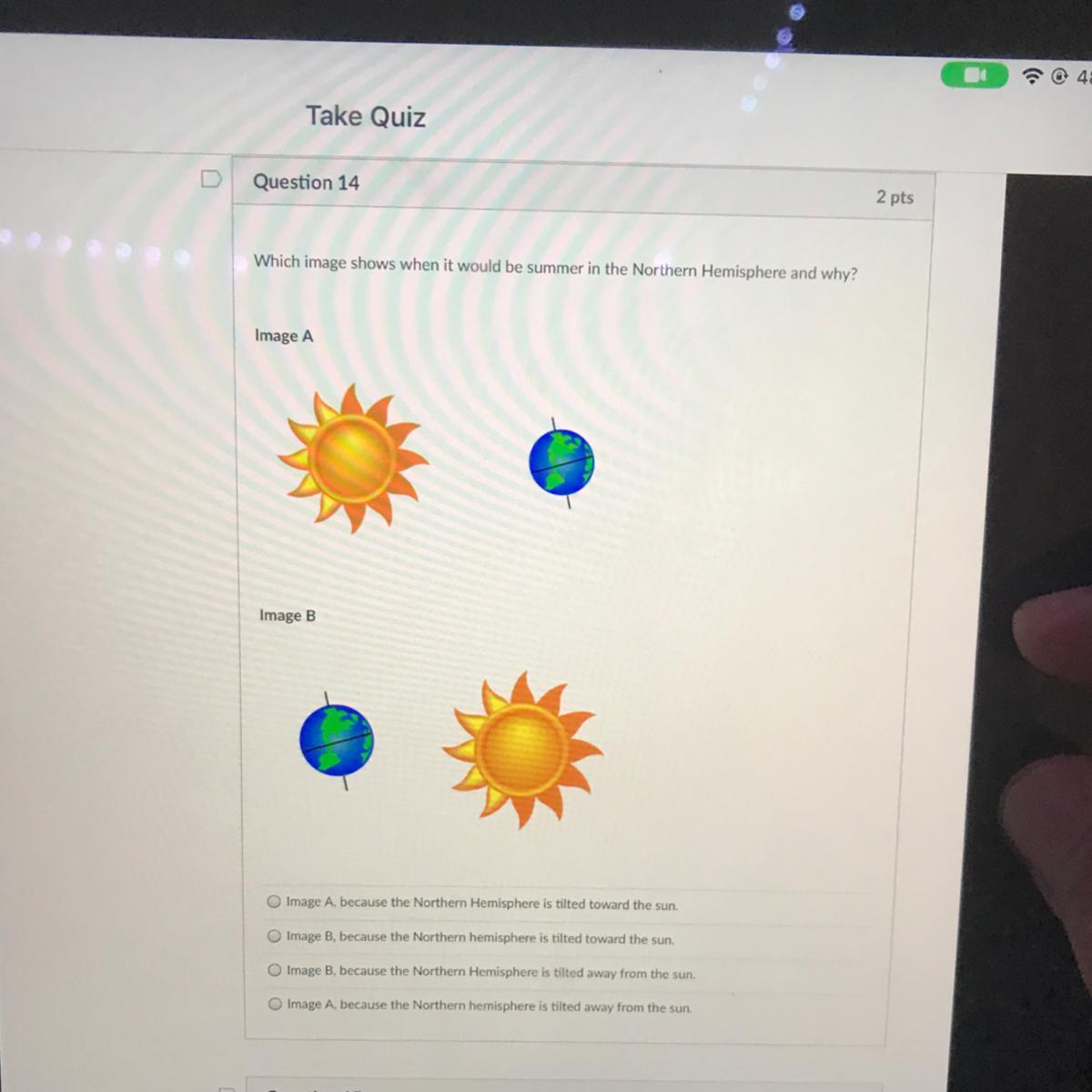
Image A
Explanation:
The hemisphere experiences summer when it is tilted towards the sun. Image a has the northern hemisphere tilted towards the sun.
Answer:
image A
Explanation:
this is because the sun is hitting earth at a steep angle
Related Questions
The chemical reaction between water and magnesium is?
Answers
Answer: hydrogen gas
Explanation: When magnesium interacts with water, it will form a hydrogen gas that ignites violently due to the excessive heat and oxygen supply.
1. Watch how the solubility of a gas changes as pressure is increased and then decreased during the run. Which of the following statements correctly explain the relationship between the solubility of a gas and its pressure?
A. As pressure decreases, the concentration of gas molecules in the solution increases.
B. The concentration of gas particles in the solution is higher at 4.25 atm than at 1.00 atm.
C. The solubility of a gas decreases with a decrease in pressure.
D. The solubility of nitrogen gas at 2.00 atm is twice the solubility of the gas at 1.00 atm.
E. Fewer gas molecules are soluble at higher pressures.
F. More gas molecules are soluble as pressure is increased.
G. As pressure is increased, the rate at which gas molecules enter the solution decreases.
2. At a certain temperature, the solubility of N2 gas in water at 3.08 atm is72.5mg of N2 gas/100 g water. Calculate the solubility of N2 gas in water, at the same temperature, if the partial pressure of N2 gas over the solution is increased from 3.08 atm to 8.00 atm .
Answers
Answer:
A. As pressure decreases, the concentration of gas molecules in the solution increases.
D. The solubility of nitrogen gas at 2.00 atm is twice the solubility of the gas at 1.00 atm.
F. More gas molecules are soluble as pressure is increased.
The solubility is 188.3 mg of N2 gas/100 g water.
Explanation:
As the pressure on the gas decreases, the volume of gas molecules in the solution increases due to having distance from each other. The solubility of nitrogen gas at 2.00 atm is twice the solubility of the gas at 1.00 atm because there is more pressure on the gas molecules so due to more compression, the gas becomes more soluble. More gas molecules are soluble as pressure is increased. The solubility is 188.3 mg of N2 gas/100 g water if the atmospheric pressure is increases from 3.08 atm to 8.00 atm. This value is calculated with the help of formula i.e. P2/P1 = S2/S1.
PLEASE HELP REAL ANSWER NO FILE. Part A
Electricity generated from any source comes with its own advantages and
disadvantages. So, no source of energy for generating electricity is perfect. However,
imagine that there is an energy source that perfectly meets the needs of society.
Describe this ideal source of energy. Include relevant factors such as cost, supply, safety,
reliability, and environmental impact
Answers
Answer:
Wind energy
Explanation:
An ideal source of energy needs to be reliable, cost effective, safe and must lead to almost zero adverse environmental impact.
Wind energy is energy obtained from air moving at high velocity. This energy is harvested using windmills which convert mechanical energy to electrical energy.
Wind is inexpensive because it occurs naturally. However, a large expanse of land is required in order to mount sufficient number of windmills that will generate enough electrical energy for practical purposes.
This method of electricity generation is safe and does not lead to any environmental hazard unlike the burning of fossil fuels, use of nuclear energy or loss of habitat due to hydroelectric power generation.
what is the difference between Earth and Exoplanets
Answers
Answer:
Earth has a strong gravitational field while exoplanets do not.
Explanation:
Rate Brainliest please
All of the planets in our solar system orbit around the Sun. Planets that orbit around other stars are called exoplanets.
Write in exponential form: 34⋅34⋅34⋅34⋅34⋅34⋅34⋅34.
Answers
Answer:
34^8
Explanation:
The electrolyte magnesium chloride (MgCl2) will break up into how many individual particles in water (what is the i value)?
Answers
Answer:
Solid magnesium chloride is a non-conductor of electricity because the ions aren't free to move. However, it undergoes electrolysis when the ions become free on melting. Magnesium chloride dissolves in water to give a faintly acidic solution (pH = or 6).
Name: ___________________________ Date: __________ Period: ______ Solubility Rules Practice Worksheet Name or give the chemical formula for each of the following compounds. State whether they are soluble (will dissolve) or insoluble (will not dissolve) in solution. Use solubility rules. Chemical Formula Name Solubility NH4OH Ra(OH)2 Nickel (III) Acetate CsOH RbCl Potassium Phosphate MgS CaI2 Gold (II) Hydroxide Li3PO4 Platinum (II) Carbonate Barium Nitrate
Answers
When considering free energy change, biochemists usually define a standard state, the biochemical standard state, which is modified from the chemical standard state to fit biochemical applications. Determine which of the phrases describe the biochemical standard state, the chemical standard state, or both.
a. Temperature is 25C
b. Initial concentration of reactants and products is 1M
Answers
Answer:
Chemical standard state
a. Temperature is 25C
b. Initial concentration of reactants and products is 1M
Biochemical standard state
a. Temperature is 25C
b. Initial concentration of reactants and products is 1M
Explanation:
The standard state is the reference state of a material which can be used to calculate its properties under other nonstandard conditions.
The biochemical standard state include;
a. Temperature is 25C
b. Initial concentration of reactants and products is 1M
Similarly, the chemical standard state include;
a. Temperature is 25C
b. Initial concentration of reactants and products is 1M
Hence the answer.
The air we breathe contains different individual gases (mostly nitrogen and oxygen). Which of the following correctly describes the air we breathe? A. mixture B. liquid C. compound D. element
Answers
Answer:
A. Mixture
Explanation:
Our air has a group of gases. For example, you said nitrogen & oxygen, Which is significantly a mixture.
please help with Chem I DON'T HAVE ENOUGH TIME!
if a 119g sample of water was allowed to evaporate completely, what volume of water vapour would be produced in milliliters?
Answers
As 1 L = 1000 g
so 119 grams = 0.119000 L
Hope it is helpful to u
If yes then plz mark me brainlest
We are given:
Mass of water: 119 grams
We know that one mole of a gas occupies 22.4L of volume
Number of moles of water:
Number of moles = given mass / Molar mass
Number of moles = 119 / 18 [molar mass of water = 18 grams/mol]
Number of moles = 6.61 moles
Volume occupied:
Volume = number of moles * 22.4 L
Volume = 6.61 * 22.4
Volume = 148L
Volume (in mL) = 1.48 * 10⁻¹ mL
What Energy transformation occurs when gasoline burns in an automobile
Answers
Answer:
The release of energy from fuels is used to make other forms of energy. When gasoline burns in a car engine, some of the chemical energy in the gasoline is converted into heat. The heat is converted into mechanical energy. The mechanical energy moves the car.I hope this helped!
Explanation:
3. At 34.0°C, the pressure inside a nitrogen-filled tennis ball with a volume of 148 cm3 is 212
kPa. How many moles of N2 are in the tennis ball?
Answers
Answer:
0.0123 mol
Explanation:
Step 1: Convert 34.0 °C to Kelvin
We will use the following expression.
K = °C + 273.15 = 34.0 + 273.15 = 307.2 K
Step 2: Convert 148 cm³ to L
We will use the conversion factors:
1 cm³ = 1 mL1 L = 1000 mL[tex]148cm^{3} \times \frac{1mL}{1cm^{3}} \times \frac{1L}{1000mL} = 0.148L[/tex]
Step 3: Convert 212 kPa to atm
We will use the conversion factor 1 atm = 101.325 kPa.
212 kPa × 1 atm / 101.325 kPa = 2.09 atm
Step 4: Calculate the moles of nitrogen gas
We will use the ideal gas equation.
P × V = n × R × T
n = P × V / R × T
n = 2.09 atm × 0.148 L / (0.0821 atm.L/mol.K) × 307.2 K = 0.0123 mol
When sodium chloride is dissolved in water, the freezing point of water _________. A. increases B. first increases, then decreases C. does not change D. decreases
Answers
The presence of a non-volatile salt will decrease the freezing point of water and this process is called depression in freezing point. Thus option D is correct.
What is freezing point?Freezing point of a substance is the temperature at which it converts from its liquid state to solid state where, both the states are in equilibrium. Freezing point of water is zero degree celsius.
The freezing point of a solvent depends on some parameters such as the bond type, molecular weight, temperature, pressure etc.
When a non-volatile solute is added to the solvent its freezing point decreases from its initial value. Because presence of non-volatile salts will affect the intermolecular attraction and thereby the energy that must be applied to freeze the compound.
Therefore, the freezing point of water decreases, when sodium chloride is added into it. Thus option D is correct.
To find more about freezing point, refer the link below;
https://brainly.com/question/2292439
#SPJ6
Disadvantages of using cisplatin as an anti-cancer drug
Answers
Answer: The following side effects are common (occurring in greater than 30%) for patients taking Cisplatin:
Nausea and vomiting. Nausea may last up to 1 week after therapy.
Low blood counts.
Kidney toxicity.
Ototoxicity hearing loss, ringing in the ears.
Blood test abnormalities (low magnesium, low calcium, low potassium)
Explanation:
My mind is ok :')
Which of the following pairs of elements could NOT react to
form an ionic compound? Check all that apply.
Answers
Answer:
Carbon and Oxygen cannot react to form an ionic compound because the two elements are non-metals. To form an ionic bond, a metal combines with a non-metal through electrostatic attraction of oppositely charged ions.
Answer:
Explanation:
The two that won't are C and O. They will react, but not ionically. O is on the left of the Periodic table and C is more or less in the middle. They form CO carbon Monoxide and CO2 which is Carbon Dioxide. They are just not ionic.
What locations are likely to be affected by an earthquake or tsunami?
San Francisco, California, USA
San Francisco, Tokyo, and Asunción
Tokyo, Japan
None of the locations
Asunción, Paraguay, South America
Answers
Answer:
All the locations can suffer earthquakes and a handful like tokyo tsunami so the question is either or so the anwer would be all
Explanation:
Which is true regarding pressure?
A.) at high pressure, the molecules are moving slower and therefore hitting other particles and the sides of a container less frequently
B.) pressure is calculated by combining the amount of space an entire object takes up.
C.) pressure is calculated by averaging the kinetic energy of all particles in a container
D.) at higher pressure indicates that particles are colliding with the walls of a container more frequently
Answers
Answer:
D
Explanation:
The higher a pressure in a system the faster the molecules within it move and collide with one another due to lack of space
How do scientists design a system?
O A. They use a system that has already been designed.
O B. They ignore influences from sources outside of the experiment.
C. They include all possible influences in their model.
D. They isolate their experiment from unwanted influences.
Answers
Answer: D
Explanation:
The scientists design a system by isolate their experiment from unwanted influences. Therefore, option D is correct.
What is system ?Chemistry's field of systems chemistry strives to understand intricate webs of interdependent molecules and their system-level characteristics. These characteristics cannot be attributed to the individual components working independently, but rather to the aggregate behavior of the system's components.
A system is a well-organized group of components that work closely together to achieve a single objective. The system receives a variety of inputs, processes those inputs through certain steps to produce specific outputs, and then combines those outputs to achieve its overall objective.
The two main categories are natural systems and designed systems. Subatomic systems, various types of biological systems, our planet, the solar system, the galactic system, and the universe are all examples of natural systems.
Thus, option D is correct.
To learn more about the system, follow the link;
https://brainly.com/question/19843453
#SPJ2
Which statements describe the types of energy emitted by the Sun? Check all that apply.
A. It emits most of its energy in gamma rays.
B. It emits all wavelengths in the electromagnetic spectrum.
C. It emits most of its energy as visible light.
D. Its peak wavelength is in the orange-yellow range.
E. It emits no X-rays or radio waves.
F. Its peak wavelength is in the yellow-green range.
Answers
Answer:
a,c,b
Explanation:
2x²=8.pls help me i really need it
Answers
Explanation:
2x²=8
x²=8/2
x=√4
x=2
hope it helps.
Answer:
[tex]\huge \fbox \pink {A}\huge \fbox \green {n}\huge \fbox \blue {s}\huge \fbox \red {w}\huge \fbox \purple {e}\huge \fbox \orange {r}[/tex]
[tex] {2x}^{2} = 8 \\ {x}^{2} = \frac{8}{2} \\ {x}^{2} = 4 \\ x = \sqrt{4} \\ x = 2[/tex]
ʰᵒᵖᵉ ⁱᵗ ʰᵉˡᵖˢ
[tex] \huge\purple{ \mid{ \underline{ \overline{ \tt ꧁❣ ʀᴀɪɴʙᴏᴡˢᵃˡᵗ2²2² ࿐ }} \mid}}[/tex]
Why do scientist conduct experiments on animals?
Answers
Answer:
Animals are used in scientific research to help us understand our own bodies and how they work. This is necessary to develop new medicines. Animals are also used to safety test potential medicines before they are tested in people and to check the safety of other chemicals.
Explanation:
Help solve the types of chemical reactions
Answers
[tex]1) \: decomposition[/tex]
[tex]2) \:hydrocarbon \: combustion[/tex]
[tex]3) \: formation[/tex]
[tex]4) \: double - replacement[/tex]
[tex]5 \: double - replacement[/tex]
[tex]6) \: formation[/tex]
[tex]7)double \: - replacement[/tex]
[tex]8) \: double - replacement[/tex]
How many grams water will condense when 56,500 joules of energy is removed from steam at its boiling point
Answers
Answer:
Start your streak by answering any question. You'll get bonus points from day 2.
The volume of a sample of carbon dioxide gas is 26.42 L at 73.0°C. What will its volume be at 92.0°C at constant pressure?
Answers
Answer:
[tex]V_2=27.87L[/tex]
Explanation:
Hello there!
In this case, according to the given information, it turns out possible for us to solve this problem by using the Charles' law a directly proportional relationship to understand the volume-temperature behavior:
[tex]\frac{V_2}{T_2} =\frac{V_1}{T_1}[/tex]
Thus, we solve for the final volume, V2, and make sure the temperature are in Kelvin as shown below:
[tex]V_2 =\frac{V_1T_2}{T_1} \\\\V_2=\frac{26.42L(92+273.15)K}{(73+273.15)K} \\\\V_2=27.87L[/tex]
Regards!
Given the reaction: N2(g) +2O2(g) ⇌ 2NO2(g) The forward reaction is endothermic. Determine which of the following changes would result in more product being produced.
I. Increase NO2
II. Decrease O2
III. Add a catalyst
IV. Increase the temperature
V. Increase the pressure
A. I and II
B. II, III, and V
C. IV and V
D. II and IV
Answers
Answer:
C
Explanation:
increasing the temperature will favour the forward reaction therefore the reaction system will try to counteract that by producing more heat and NO2 therefore increases the amount of products produced
increasing the pressure will favour the forward reaction as it has more moles of substance therefore if the forward reaction is favored, more product will be produced
Answer:
C.) lV and V
Explanation:
I got it correct on founders edtell
n today's experiment, Solutions A and B are prepared as follows. Solution A: Solution B: 2.0 mL of 3.00 x 10-4 M bromcresol green 2.0 mL of 3.00 x 10-4 M bromcresol green 5.0 mL of 1.60 M acetic acid (HAc) 2.0 mL of 0.160 M sodium acetate (NaAc) 2.0 mL of 0.200 M KCl diluted to a total volume of 50 mL diluted to a total volume of 50 mL How many mL of Solution A must be added to Solution B to give a buffer that is equimolar in HAc and Ac-
Answers
Answer:
2 mL of Solution A must be added to Solution B to give a buffer that is equimolar.
Explanation:
Given the data in the question;
First we determine the number of sodium acetate;
⇒ molarity × volume ( L )
⇒ 0.16 × 2.0 mL
⇒ 0.16 × 0.002 L
⇒ 0.00032
Now, Molarity of sodium acetate = moles / Volume(L)
⇒ ( 0.00032 / 50 ) × 1000
⇒ 0.0064
Since number of moles of acetic acid that should be added tp make equimolar solution is 0.00032
and Molarity of acetic acid is 0.16 molL⁻¹
Let X represent the volume that should be added.
so;
Molarity = Moles / Volume (L)
we substitute
0.16 = (0.00032 / X) × 1000
0.16 = 32 / X
X = 0.32 / 0.16
X = 2 mL
Therefore, 2 mL of Solution A must be added to Solution B to give a buffer that is equimolar.
please help- science
1. - new
2. full
3. first quarter
4. last quarter
Answers
Answer:
2. Full
Explanation:
A lunar eclipse occurs at a full moon when Earth is directly between the moon and the sun. During a lunar eclipse, Earth blocks sunlight from reaching the moon.
Hope this helps!!
The addition of dimethylglycoxime, H2C4H6O2N2, to a solution containing nickel(II) ion gives rise to a precipitate: Ni2 2H2C4H6O2N2 Ni(H2C4H6O2N2)2 2H If 0.15 g nickel alloy is treated with dimethylglycoxime and .175 mg nickel dimethylglycoxime is collected. Determine the mass and percent of nickel in the alloy.
Answers
Solution :
The balanced equation is :
[tex]$Ni^{2+}+2H_2C_4H_6O_2N_2 \rightarrow Ni(H_2C_4H_6O_2N_2)_2+2H^+$[/tex]
Molar mass 56.7 116 290.7
From the balanced equation,
2 mole
= 2 x 116 g of [tex]$H_2C_4H_6O_2N_2$[/tex] produces 1 mole = 290.7 g of nickel dimethylglycoxime
or 2 x 116 mg of [tex]$H_2C_4H_6O_2N_2$[/tex] produces 1 mole = 290.7 g of nickel dimethylglycoxime
0.175 mg of [tex]$H_2C_4H_6O_2N_2$[/tex] produces [tex]$\frac{0.175 \times 290.7}{2 \times 116}$[/tex] = 0.219 mg of nickel dimethylglycoxime
290.7 g of [tex]$Ni(H_2C_4H_6O_2N_2)_2$[/tex] contains 58.7 mg of Ni
0.219 mg of [tex]$Ni(H_2C_4H_6O_2N_2)_2$[/tex] contains [tex]$\frac{0.219 \times 58.7}{290.7} = 0.0443$[/tex] mg of Ni
So mass of nickel, m = 0.0443 mg = [tex]$0.0443 \times 10^{-3}$[/tex] g
Percent of Nickel in the alloy = [tex]$\frac{\text{mass of nickel}}{\text{mass of alloy}} \times 100$[/tex]
[tex]$=\frac{0.0443 \times 10^{-3}}{0.159}\times 100$[/tex]
= 0.03%
What happens to iron when it melts?
O A. It undergoes a chemical change.
O B. It undergoes a physical change.
O c. Its atoms break apart and form new atoms.
O D. Its atoms combine and form new atoms.
Answers
Answer:
B. It undergoes a physical change.
Explanation:
Hello there!
In this case, since we know that chemical changes lead to the formation of new atoms and/or compounds due to the bonds rearrangement of the initial substances and the physical changes occur without changing the composition of the initial substances, we can infer that, since melting is a process that changes the phase of matter from solid to liquid without changing the identity of the initial substance, the answer to this question would be B. It undergoes a physical change. because the other options describe chemical changes.
Best regards!
Answer:
B. It undergoes a physical change.
Explanation:
At room temperature, the iron atoms are in an unusual loosely packed open arrangement; as iron is heated past 912 degrees Celsius, the atoms become more closely packed before loosening again at 1,394 degrees Celsius and ultimately melting at 1,538 degrees Celsius.
ch3-co-ch2-ch2-ch3 IUpAC name
Answers
Answer:
2-pentanone.
Explanation:
Hello there!
In this case, for the given compound and, in agreement with the octet rule, it is possible to realize that the CO is actually C=O as shown below:
CH3 - C - CH2 - CH2 - CH3
||
O
Thus, since the C=O stands for the carbonyl group within the parent chain, we infer this is a ketone and more specifically 2-pentanone as it has five carbon atoms.
Regards!