Answers
Explanation:
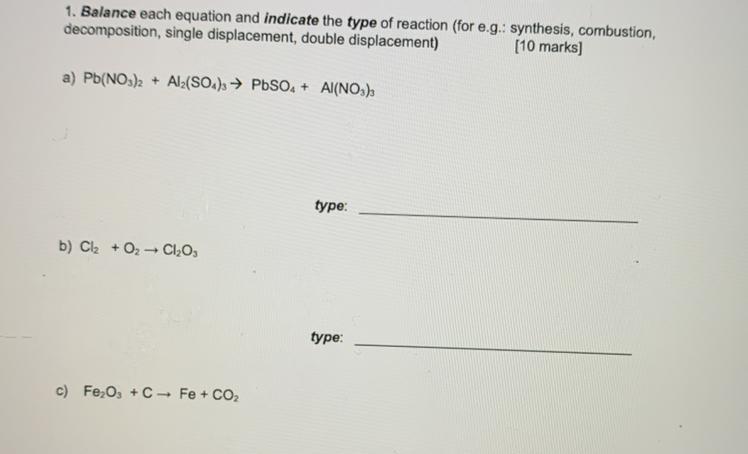
a) 3Pb(NO3)2 + Al2(SO4)3 ---> 3PbSO4 + 2Al(NO3)3
Double displacement
b) 2Cl2 + 3O2 ---> 2Cl2O3
Synthesis
c) 2Fe2O3 + 3C ---> 4Fe + 3CO2
single displacement
Related Questions
Potassium hydrogen phthalate is a solid, monoprotic acid frequently used in the laboratory as a primary standard. It has the unwieldy formula of KHC8H4O4. This is often written in shorthand notation as KHP. If 31.0 mL of a barium hydroxide solution are needed to neutralize 1.37 grams of KHP, what is the molarity of the barium hydroxide solution
Answers
Answer:
0.1082M of Barium Hydroxide
Explanation:
KHP reacts with Ba(OH)2 as follows:
2KHP + Ba(OH)2 → 2H2O + Ba²⁺ + 2K⁺ + 2P²⁻
Where 2 moles of KHP reacts per mole of barium hydroxide
To solve this question we must find the moles of KHP in 1.37g. With these moles and the reaction we can find the moles of Ba(OH)2 and its molarity using the volume of the solution (31.0mL = 0.0310L) as follows:
Moles KHP -Molar mass: 204.22g/mol-
1.37g * (1mol / 204.22g) = 0.006708 moles KHP
Moles Ba(OH)2:
0.006708 moles KHP * (1mol Ba(OH)2 / 2mol KHP) =
0.003354 moles Ba(OH)2
Molarity:
0.003354 moles Ba(OH)2 / 0.0310L =
0.1082M of Barium HydroxideBase your answer on the equation and diagram below represent an electrochemical cell at 298 K and 1 atmosphere.
When the switch is closed, electrons flow from
A) Ag+(aq) to Mg2+(aq)
B) Mg(s) to Ag(s)
C) Ag(s) to Mg(s)
D) Mg2+(aq) to Ag+(aq)
Answers
When the switch is closed, electrons flow from the solid magnesium electrode, Mg(s) to solid silver electrode, Ag(s).
Electronegativity of metals
Electronegativity of metals refers to the ability of the atoms of metallic elements to attract electrons from the other metallic elements.
Electronegativity increases down the activity series.
Silver (Ag) will have more tendency to attract electron more than magnesium (Mg).
Thus, when the switch is closed, electrons flow from the solid magnesium electrode, Mg(s) to solid silver electrode, Ag(s).
Learn more about electronegativity here: https://brainly.com/question/24977425
#SPJ2
g A chemical equilibrium exists when: A chemical equilibrium exists when: there are equal amounts of reactants and products. the rate at which reactants form products is the same as the rate at which products form reactants. the sum of reactant and product concentrations equals one mole. reactants are completely changed to products. the rate at which reactants form products becomes zero.
Answers
Answer:
the rate at which reactants form products is the same as the rate at which products form reactants
Explanation:
There is still a reaction happening just that the second one happens the opposite happens and keeps it at net 0
i think its D but i not sure so can yall pls help me
Answers
Answer:
I'm not really sure but I thinks its
A:veuns
Which statement best describes thermodynamics?
A. The study of how energy changes and moves
B. The study of physical laws acting on matter
C. The study of electricity at low temperatures
D. The study of intermolecular forces in liquids
Answers
Answer:
a
Explanation:
PLZ HELP ON TIMER WILL GIVE BRAINLIEST
How does technology limit the future of space exploration?
There are too many devices in space interfering with taking correct measurements.
Scientists cannot make contact with older satellites in outer space.
Scientists are able to work both with current and future technology.
Scientists must first develop certain technologies before missions can be completed.
Answers
Answer:
I would put the final answer choice: "Scientists must first develop certain technologies before missions can be completed"
Explanation:
The first option is partially true, but we have ways around it.
The second option is straight-up false.
The third option doesn't make much sense, how can one work with technology that will be developed in the future and doesn't yet exist?
Therefore, the fourth option is the best.
Hope this helps
-cyber
In an effort to maintain homeostasis, the organ systems of the human body work to keep the body's internal water level constant. Which of the following statements describe how the human body responds when its internal water level drops too low?
A. The skeletal system produces more circulating blood cells that insecure the body’s water intake
B. The excretory system signals the kidneys to retain more water and produce more concentrated urine to decrease water loss.
C. The nervous system signals the muscles to constrict , holding more water in the digestive track and decreasing water loss .
D.The excretory system signals the kidneys to release more water into the bladder to increase water loss .
Answers
The rate of reaction was measured during a chemical reaction. After the first 3 seconds, the rate of reaction was 1.8 x10−6 M/s. Which of the following would you expect after another 3 seconds?
Answers
The question is incomplete, the complete question is;
The rate of reaction was measured during a chemical reaction. After the first 3 seconds, the rate of reaction was 1.8 x10−6 M/s. Which of the following would you expect after another 3 seconds? a
The rate would be higher, and the concentration of reactants would be lower.
b
The rate would be higher, and the concentration of reactants would be higher.
c
The rate would be lower, and the concentration of reactants would be lower.
d
The rate would be lower, and the concentration of reactants would be higher.
Answer:
The rate would be lower, and the concentration of reactants would be lower.
Explanation:
The rate of reaction refers to how quickly or slowly the reactants disappear or the products appear in a given reaction. The rate of reaction depends on the concentration of the reactants. Thus, as concentration decreases with time, the rate of reaction decreases accordingly.
Therefore, reaction rates tend to decrease with time since the concentration of the reactants decrease with time as the reactants are being converted into products. Thus after three seconds, the rate would be lower, and the concentration of reactants would be lower. Hence the answer above.
Answer:
The rate would be lower, and the concentration of reactants would be higher.
Explanation:
I took the test and i think i got it right
Please help will give brainliest
Perform the following
mathematical operation, and
report the answer to the correct
number of significant figures.
328 x 0.125 = [?]
Answers
Answer: 41.0
Explanation: When you multiply the two numbers you get 41 but you need to have the same amount of significant numbers as the number in the problem with the least significant numbers. I hope this helps
1. What is the kinetic energy of a cow that has a mass of 1500 kg and is moving at 4
meters per second?
Answers
Answer:
12000j
mass=1500kg
velocity=4m/s
Kinetic Energy (K E) = 1/2 mv^2
=1/2*1500(4^2)
=1*750*16
=12000j
please help ME WITH CHEM I DONT HAVE ENOUGH TIME! calculate the number of hydrogen molecules that it would have contained at stp if it had a volume of 200,00m cubed.
Answers
We know that, at STP, one mole of a gas occupies 22.4 L of volume
we'll use the same principle to solve this problem
Converting given volume to Liters:
We know that 1 m³ = 1000 L
So, 20000 m³ = (20000)*(1000) L = 2 * 10⁷ L
Converting Liters to moles:
As mentioned above, at STP, one mole occupies 22.4 Liters
Number of moles in 2 * 10⁷ L = 2 * 10⁷ / 22.4
Number of moles = 8.9 * 10⁵ moles
Converting moles to number of particles:
We know that 1 mole contains 6.022 * 10²³ molecules
So, 8.9 * 10⁵ moles contain [(8.9 * 10⁵) * (6.022 * 10²³)] molecules
Number of molecules = 53.6 * 10²⁸ molecules
In proper scientific notation:
Number of molecules = 5.36 * 10²⁹ molecules
Help plz:)))I’ll mark u Brainliest
Answers
Answer:
82gS
Explanation:
2 Fe + 3 S → Fe2S3
↓ ↑
mol → mol
Fe S
[tex]\frac{95 g Fe }{} \frac{1 mol Fe }{55.85 g Fe } \frac{3 mol S }{2 mol Fe } \frac{32.07 g S}{1 mol S} = 82 g S[/tex]
Draw the most stable form of the major product in the reaction. 2 equivalents of an ester react with N a O C 2 H 5, followed by H 3 O plus and C 2 H 5 O H. The ester is a carbonyl bonded to a 3 carbon chain and O C H 2 C H 3.
Answers
Answer:
See answer below
Explanation:
This is a Claisen condensation. In the picture below you have the mechanism and final product.
Hope this helps
Calculate the Ka of a 0.35M weak acid with a pH of 4.2.
Answers
Answer:
Ka = 1.14x10⁻⁸
Explanation:
First we calculate [H⁺] from the pH:
pH = -log[H⁺][H⁺] = [tex]10^{-pH}[/tex][H⁺] = 6.31x10⁻⁵ MFor a monoprotic weak acid, the molar concentration of H⁺ of a solution can be expressed as:
[H⁺] = √(C*Ka)Where C is the molar concentration of the weak acid solution.
6.31x10⁻⁵ M = [tex]\sqrt{0.35M*Ka}[/tex]1.14x10⁻⁸ = KaA gas has a solubility of 2.45 g/L at a pressure of 0.750 atm. What pressure wold be required to produce an aqueous solution containing 6.25 g/L of this gas at constant temperature
Answers
Answer:
1.91 atm
Explanation:
Step 1: Calculate Henry's constant (k)
A gas has a solubility (C) of 2.45 g/L at a pressure (P) of 0.750 atm. These two variables are related to each other through Henry's law.
C = k × P
K = C/P
K = (2.45 g/L)/0.750 atm = 3.27 g/L.atm
Step 2: Calculate the pressure required to produce an aqueous solution containing 6.25 g/L of this gas at constant temperature.
We have C = 6.25 g/L and k = 3.27 g/L.atm. The required pressure is:
C = k × P
P = C/k
P = (6.25 g/L)/(3.27 g/L.atm) = 1.91 atm
If a particular ore contains 56.3 % % calcium phosphate, what minimum mass of the ore must be processed to obtain 1.00 kg k g of phosphorus?
Answers
Answer:
34.44 kg
Explanation:
First we convert 1.00 kg of phosphorus (P) into moles, using its molar mass:
1.00 kg ÷ 32 kg/kmol = 0.03125 kmol PThen we convert 0.03125 kmoles of P into kmoles of Ca₃(PO₄)₂:
0.03125 kmol P * [tex]\frac{2kmolCa_3(PO_4)_2}{2kmolP}[/tex] = 0.0625 kmol Ca₃(PO₄)₂Now we calculate the mass of 0.0625 kmoles of Ca₃(PO₄)₂:
0.0625 kmol Ca₃(PO₄)₂ * 310.18 kg/kmol = 19.39 kgFinally we calculate the required mass of the ore, using the definition of content percentage:
% content = Mass of calcium phosphate / mass of ore * 100 %56.3 % = 19.39 kg / mass of ore * 100%Mass of Ore = 34.44 kgSelect the correct answer.
Using this activity chart, which reaction will happen when a piece of copper is placed in a lead nitrate solution?
A.
2Cu + 3Pb(NO3)2 3Cu(NO3)2 + 2Pb
B.
No reaction occurs.
C.
2Cu + 3Pb(NO3)2 2Cu(NO3)2 + 3Pb
D.
3Cu + 3Pb(NO3)2 3Cu(NO3)2 + 3Pb
E.
The answer cannot be determined from the information given.
Answers
Answer:
B, No reaction will occur
Explanation:
Copper as compared to lead is less reactive. This is the reason when lead is added to copper nitrate solution, it replaces the copper and itself combines with nitrate to form lead nitrate aqueous solution
Lead + Copper(II) nitrate → Copper + Lead (II) nitrate
The same is not the case when the reaction is revered i.e Cu is added to Pb NO3 solution.
Hence, option B is correct
PLEASE HELP EMERGENCY ‼️‼️ needed ASAP
Answers
Answer:
the photo is kind of blurry i cant really see it, sorry
Explanation:
1. How many grams of sodium carbonate must be weighed out in order to make 2.5 kg of a 35.0%
(w/w) solution?
Answers
Answer:
... x M x V. • Example: Prepare 800 mL of 2 M sodium chloride. ... solutions are indicated by w/v% and are defined as the grams of solute per ...
15 pages·906 KB
Which of the following statements explains how concentration of a solution affects the rate of a reaction? (5 points)
O a
When concentration decreases, kinetic energy increases.
Ob
When concentration increases, particles collide less often.
When concentration increases, particles collide more often.
Oc
Od
When concentration decreases, collision of particles increases.
Answers
Answer:
when concentration increases, particles colide more often
The statement "When concentration increases, particles collide more often" is correct.
What is concentration?A constituent's abundance divided by the entire volume of a combination is known as concentration. Mass concentration, molar concentration, number concentration, and volume concentration are all examples of mathematical descriptions.
There are much more reactant particles travelling together as the concentration of reacting species increases. Because there would be more collisions, the reaction rate will be faster. The rate of a reaction increases as the concentration of reactants increases.
To know more about concentration click here.
https://brainly.com/question/10725862.
#SPJ2
Match the solutions to the descriptions of the freezing points.
a. One mole of the ionic compound Na3PO4 dissolved in 1000 g H2O
b. One mole of the ionic compound CuSO4 dissolved in 1000 g H2O
c. One mole of the nonelectrolyte C6H12O6 dissolved in 1000 g H2O
1. Highest freezing point
2. Intermediate freezing point
3. Lowest freezing point
Answers
Answer:
1c
2b
3a
Explanation:
Step 1: Write the general expression to calculate the freezing point depression
The freezing point depression is a colligative property, that can be calculated using the following expression.
ΔT = i × Kc × b
where,
i: van 't Hoff factor (number of ion particles per formula unit)
Kc: cryoscopic constant (Kc for water: 1.86 °C.Kg/mol)
b: molality (moles of solute per kilogram of solvent)
All the solution have the same Kc and the same b (1 mol/1 kg = 1 m), so ΔTf variation will depend on i
Step 2: Calculate the freezing point of the Na₃PO₄ solution
Na₃PO₄ has 4 ions (3 Na⁺ and 1 PO₄³⁻), so i = 4.
ΔT = i × Kc × b = 4 × 1.86 °C.Kg/mol × 1 mol/kg = 7.44 °C
T = 0°C - 7.44 °C = -7.44 °C
Step 3: Calculate the freezing point of the CuSO₄ solution
CuSO₄ has 2 ions (1 Cu²⁺ and 1 SO₄²⁻), so i = 2.
ΔT = i × Kc × b = 2 × 1.86 °C.Kg/mol × 1 mol/kg = 3.72 °C
T = 0°C - 3.72 °C = -3.72 °C
Step 4: Calculate the freezing point of the C₆H₁₂O₆ solution
C₆H₁₂O₆ is a nonelectrolyte (it doesn't ionize), so i = 1
ΔT = i × Kc × b = 1 × 1.86 °C.Kg/mol × 1 mol/kg = 1.86 °C
T = 0°C - 3.72 °C = -1.86 °C
How many moles of nitrogen are required to produce 13.5 g of NH 3?
Answers
Answer:
number of moles of (N) = 0.794 moles
Explanation:
From the given information:
no of moles of nitrogen (N) = (unknown)???
mass of nitrogen = 13.5 g
molar mass of NH3 = 14 +( 1 × 3) = 17 g/mol
To calculate the no of moles of N, we have:
number of moles of (N) = mass of N/molar mass
number of moles of (N) = 13.5 g/17 g/mol
number of moles of (N) = 0.794 moles
How many moles are in 153 grams of O2?
Answers
Answer:
4.78 mol O2
Explanation:
Grams --> moles
Grams/molar mass = moles
153 g O2 / 32 g/mol O2 = 4.78125 (significant figures so round it up; the grams has 3 significant figures, so round it up) 4.78 mol O2
Que uso le darías a la vida diaria escribe en español plis
Answers
Explanation:
Facilitar el movimiento y desplazamiento de objetos pesados reduciendo el rose del objeto contra el suelo, Obtener un movimiento circular por efecto de la fuerza de un liquido, en el caso de contadores, molinos, centrales hidroeléctricas y turbinas.
Transmitir el movimiento de un eje a otro como en el caso de las lavadoras, bicicletas y neveras. Reducir drásticamente el esfuerzo necesario para elevar y mover objetos, como en casos de pozos de agua y ascensores Transformar movimientos giratorios en otros movimientos o viceversa. ,
True or False: A higher vapor pressure (evaporates easily) corresponds to strong intermolecular forces.
Answers
How long does it take to fill one train car full of nuclear power can keep a power plant running
Answers
What are the components of DNA?
A. ribose sugar, cytosine, guanine, adenine, thymine, and phosphate group
B. ribose sugar, cytosine, guanine, adenine, uracil, and phosphate group
C. deoxyribose sugar, cytosine, guanine, adenine, thymine, and phosphate group
D. deoxyribose sugar, cytosine, guanine, adenine, uracil, and phosphate group
Answers
Answer:
C
Explanation:
A-T G-C
Answer:
C
Explanation:
EDGE 2022
Why doesnt the gas on a gas giant escape into space, as it has on mercury?
Answers
Answer:
When surface gravity is ore than the escape velocity gases can not escape from the planet.
Explanation:
Mercury is near Sun and the solar wind is also blowing out the atmosphere.
The gas on a gas giant escape into space, as it does on mercury because mercury is closest to sun.
What is a gas giant?A gas giant is defined as a giant planet which is composed mainly of elements of hydrogen and helium. Gas giants are also called failed stars because they contain the same basic elements as a star. Jupiter and Saturn are the gas giants of the Solar System. The term "gas giant" was originally synonymous with "giant planet". However, in the 1990s, it became known that Uranus and Neptune are really a distinct class of giant planets, being composed mainly of heavier volatile substances.For this reason, Uranus and Neptune are now often classified in the separate category of ice giants.
Jupiter and Saturn consist mostly of elements of hydrogen and helium, with heavier elements making up between 3 and 13 percent of their mass. They are thought to consist of an outer layer of compressed molecular hydrogen surrounding a layer of liquid metallic hydrogen, with probably a molten rocky core inside.
Learn more about gas giant,here:
https://brainly.com/question/9401838
#SPJ2
How is an exothermic reaction indicated in an equation?
Answers
[tex] \longrightarrow\sf \pmb {Exothermic \: Reaction}[/tex]
[tex] \tt \green {An \: exothermic \: reaction \: is \: indicated \: by \: writing \: “+Heat” \: or \: “+Heat \: energy” \: or \: “+Energy” \: on \: the \: products \: side \: of \: an \: equation.}[/tex]
Answer: Heat is included as one of the products.
Explanation:
(NH4)2Cr2O7 Cr2O3 + N2 + H2O
If 4.7369 moles of H2O are produced, how many moles of (NH4)2Cr2O7 were reacted?
Answers
Answer:
the original substances in any chemical reaction. products. the resulting substances in any....chromium(III) oxide, and water. (NH4)2Cr2O7(s) → N2(g) + Cr2O3(s) + 4H2O(g).