Answers
Explanation:
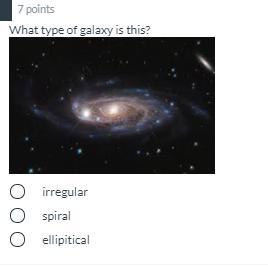
it is an spiral as it are rounded so
It is a spiral type of galaxy.
Related Questions
a student is trying to classify an unidentified, solid gray material as a metal or a non metal. which question will best help the student classify the material?
F. Is the material malleable or ductile?
G. Does the material feel hard to the touch?
H. Will the material float in water?
J. Does the material feel rough or smooth?
Answers
Answer:
i think the answer is f as metals are malleable and ductile
Answer:
F
Explanation: Most metals carry the trait that they are malleable and ductile.
Given the balanced equation below, how many moles of chromium are
produced? 2Cr2O3 + 3S1 --> 4Cr + SIO3
O A. 1 mole
B. 2 moles
O C. 3 moles
O D. 4 moles
Answers
Does liquid detergent conduct electricity?
Answers
Answer:
Yes, it is a good conductor of electricity.
Explanation:
They allow electric particles to pass through them (the bases).
(Hope this made sense)
can someone explain in detail how molar mass, Avogadro's number, and volume are all connected through moles? Im so confused :(
Answers
Answer:
See Explanation
Explanation:
By definition, 1 mole is the mass of substance (or, formula mass in grams) containing 1 Avogadro's Number (N₀ = 6.02 x 10²³) of particles. That is ...
1 mole of hydrogen atoms (H) = 1.00794 grams
1 mole of molecular hydrogen (H₂) = 2.01588 grams
1 mole of any substance = 1 formula weight in grams
1 mole = 1 Avogadro's Number (N₀) = 1 formula weight in grams
In the concept of 'gas laws' 1 mole of any (all) gas at STP conditions ( => 0°C & 1 atmosphere pressure) occupies 22.4 Liters & is known as the 'molar volume' of a gas at STP. If the temperature &/or pressure change the volume will not be 22.4 Liters.
For reactions whose coefficients are balanced to the lowest whole number values (i.e., no fractional coefficients) the equation is known as the 'standard reaction' and conditions are assumed to be STP and the coefficients of gas phase components indicate molar volumes. Example ...
Given N₂(g) + 3H₂(g) => 2NH₃(g) is assumed to be at 0°C; 1 Atm pressure.
Molecular Nitrogen = 1 molar volume = 22.4 Liters of N₂(g)
Molecular Hydrogen = 3 molar volumes = 3 x 22.4 Liters of H₂(g) = 67.2 Liters of H₂(g)
Molecular Ammonia = 2 molar volumes = 2 x 22.4 Liters of NH₃(g) = 44.8 Liters of NH₃
6CO2 + 6H20 → C,H120, + 602
6. How many molecules of CO2 are presenta
7. How many atoms of oxygen are present in the reactants?
8. How many atoms of carbon are present in the products
9. How many total atoms are present in C.H120,?
10. Is the equation above balanced or unbalanced?
Answers
Explanation:
The equation of the reaction is given as;
6CO2 + 6H2O → C6H12O6 + 6O2
How many molecules of CO2 are present?
6 moles
How many atoms of oxygen are present in the reactants?
(6 * 2) + (6 * 1 ) = 12 + 6 = 18
How many atoms of carbon are present in the products?
(6 * 1 ) = 6
How many total atoms are present in C6H12O6?
6 + 12 + 6 = 24 atoms
Is the equation above balanced or unbalanced?
This is a balanced equation since the number of atoms of the elements is the same in the reactant and products.
Hi hello I need help ASAP
Determine the slope of this line
a.2.5
b.0.75
c.1
d.0.4
Answers
Answer:
so I got it wrong the first time but you should pick two points on the line such as 4,3 and 9,5. Then use the equation y^2 - y^1/ x^2 - X^1. You would get 2/5 which is D.
Explanation
A reaction is expected to produce 28.3 moles of hydrogen gas. If the hydrogen is collected at 297 K and 1.08 atm, what is the volume? 305 L H2 639 L H2 948 L H2 1,240 L H2
Answers
Answer:
The correct answer is 639 L H₂
Explanation:
We use the ideal gas equation:
We have the following data:
n = 28.3 mol
T= 297 K
P= 1.08 atm
R= 0.082 L.atm/K.mol (gas constant)
We introduce the data in the gas equation to calculate the volume (V):
PV=nRT
⇒V =nRT/P = (28.3 mol x 0.082 L.atm/K.mol x 297 K)/(1.08 atm) = 638.2 L ≅ 639 L
Therefore, the correct option is 639 L H₂
Answer:
639 L H2
Explanation:
took the quiz
ur welcome
Chlorine atom
10. What does the following equation represent; 2KMnO4 -> K2MnO4 + MnO2(s)
+ O2(g) ?*
A. Oxygen turned in to carbon dioxide
B. Sulphate and lithium boiled
C. Decomposition of potassium permanganate(Heating)
D. None of the above
Answers
Answer:
C. Decomposition of potassium permanganate(Heating)
Explanation:
The equation of the reaction is given as;
2KMnO4 -> K2MnO4 + MnO2(s) + O2(g)
Reactant = 2KMnO4
Products = K2MnO4 + MnO2(s) + O2(g)
A. Oxygen turned in to carbon dioxide
Incorrect option - Oxygen is not the reactant
B. Sulphate and lithium boiled
Incorrect option - Sulphate and lithium are not part of this reaction
C. Decomposition of potassium permanganate(Heating)
Correct option - potassium permanganate decomposed to form K2MnO4 + MnO2(s) + O2(g)
D. None of the above
Incorrect option
9. What does C12 (g) represents?
A. Chlorine ions
B. Chlorine liquid
C. Chlorine gas
D. Chlorine atom
Answers
Answer:
chlorine gas since it exists as a diatomic molecule
1CaC2 + 2O2 ---> 1Ca+2CO2
I need the mole to mole ratio.
Answers
Which statement is not
true?
A. The troposphere is responsible for nearly
all of Earth’s weather.
B. The exosphere reaches deep into space
and is the least dense layer.
C. The thermosphere typically breaks up
meteors before they hit Earth.
D. The stratosphere allows commercial
airlines to fly with less turbulence because
of fewer convection currents.
Answers
explanation: to make C correct, the sphere that breaks up meteors is the mesosphere.
The atmosphere of the earth is layered and each layer of the atmosphere has its own properties. The atmosphere of the earth is divided into four layers. The thermosphere typically breaks up meteors before they hit Earth is not correct. The correct option is C.
What is mesosphere?The four important layers of atmosphere are troposphere, stratosphere, thermosphere and mesosphere. The layer of the atmosphere which is found above the stratosphere is defined as the mesosphere. It is the coldest layer of the atmosphere.
A meteor is known as a celestial object which is made up of rocks and minerals which enters the atmosphere of the earth and burns out completely before reaching the surface of the earth. It is known as the shooting star or falling star.
The temperature of mesosphere drops with altitude. By 80 km it reaches 100 degree celsius. The meteors burn up in this layer not in thermosphere.
Thus the correct option is C.
To know more about earth layers, visit;
https://brainly.com/question/13497783
#SPJ3
Calculate the molarity of a solution that contains 85.0 g of Zn(C2H3O2)2 in 250. mL of solution (don't forget to convert mL to L first). Round to the nearest hundredth.
Answers
Answer:
[Zn(C₂H₃O₂)₂] = 1.85M (3 sig-figs accurate to 0.01 Molar)
Explanation:
Concentration is defined as amount of solute in a specified volume of solution. That is, Concentration = mass of solute/volume of solution (solution = solute + solvent). When concentration is in terms of Molar values the essential relationship is Molarity(M) = moles solute / volume of solution in liters.
For this problem => first, convert mass of Zn(C₂H₃O₂)₂ into moles and then the volume of solution into liters. Take ratio of moles/volume of solution (L).
=> Molar Concentration = moles of Zn(C₂H₃O₂)₂ / Liters of solution
moles of Zn(C₂H₃O₂)₂ = 85.0 grams Zn(C₂H₃O₂)₂ / formula weight of Zn(C₂H₃O₂)₂ = 85.0g/183.5g·mole⁻¹ = 0.463 mole Zn(C₂H₃O₂)₂.
Volume of solution in Liters = 250ml / 1000ml/L = 0.250 liters
Molarity of Zn(C₂H₃O₂)₂ solution = 0.463 mole Zn(C₂H₃O₂)₂ /0.250 liters of solution = 1.85 Molar in Zn(C₂H₃O₂)₂
_____________
Note: The symbiology convention for 'molar solution concentration' is to place brackets around the molecular formula. That is, [Zn(C₂H₃O₂)₂] = 1.85M
Helppp me pretty please
Answers
Answer:
I think the correct answer would be the second one
Calculate the number of moles of NaOH contained in 250. mL of a 0.05M solution?
Answers
Answer:
0.0125 moles of NaOH are present
Explanation:
Molarity, M, is an unit of concentration widely used in chemistry. Is defined as the ratio between moles of solute (In this case, NaOH) and liters of solution.
250.0mL are = 0.250L of solution. As the molarity of the solution is 0.05M = 0.05moles / 1L, the moles present are:
0.250L * (0.05moles / 1L) =
0.0125 moles of NaOH are present2NO+2CO N 2 +2CO 2 There are 3.0 g of NO and 3.0 g of CO reacting . What is the limiting reactant ?
Answers
Answer: NO
Explanation:
As per the given balanced equation, 2 moles of NO reacts with 2 moles of CO. Hence, 3 g or 1.07 moles of CO needs to react with the same number of moles of NO. But we have only 3 g or 0.1 mole of NO. Hence, NO is the limiting reactant.
What is limiting reactant ?A limiting reactant in a reaction is the reactant which is fewer in amount or consume early without complete reaction with other reactants. Hence, as soon as this reactant is consume, the reaction stops.
Given 2 moles of NO reacts with 2 moles of CO.
molar mass of NO = 30 g/mol
molar mass of CO = 28 g/mol.
Number of moles in 3 g of CO = 3/28 = 1.07 moles
number of moles in 3 g of NO = 3/30 = 0.1 moles.
1.07 moles of CO needs 1.07 moles of NO. But only 0.1 moles are present.
Therefore, NO is the limiting reactant in this reaction.
Find more on limiting reactants:
brainly.com/question/14225536
#SPJ2
why is atomic radi measured using the nuclues from 2 of the same atoms
Answers
Explanation:
Atomic radii is measured using the distance between nucleus of 2 atoms rather than the distance between the nucleus and outermost shell because:
- There is no clear/sharp boundary of the orbital. This is why it is called an electron cloud.
- Also, the exact location of the electron is not known. What is known is the probability of finding the electron there.
Due to this, it is not possible to measure the atomic radii precisely. That's why the distance between the nucleus of two atoms is used.
A 30.0 mL aliquot of 0.0250 M HCl solution is titrated using 0.0500 M NaOH.
A) determine the volume of NaOH necessary and the pH at the equivalence point.
B) Determine the pH of the solution after 160.00 mL base is added.
Answers
Answer:
xdyd jdkdudktvko?ñhixiskrsjdjuxguxhicjovyx bhfoggu vohudixiyd siydyoxoyxyoxyixhoxiyxys ztiziyxyoxoyzyizitztixtx no se
hkgkfu
Explanation:
iyxyx yo gkxtuztizyidtiztizigxigxkhxhk. jvkgxitxoyfihciyxyixihxoyxyodyidtistusdtidti lo siento
A mixture of large, dispersed particles.
Answers
Answer:
Colloids are mixtures in which one or more substances are dispersed as relatively large solid particles or liquid droplets throughout a solid, liquid, or gaseous medium.
Answer:
Colloids are mixtures in which one or more substances are dispersed as relatively large solid particles or liquid droplets throughout a solid, liquid, or gaseous medium.
Explanation:
I got the answer first
PLEASE HELP ME.!
This illustrates which stage of meiosis?
A) Anaphase II
B) Metaphase II
C) Prophase II
D) Telophase II
Answers
Answer:
Anaphase II
Explanation:
How many water molecules are in 5.2 moles of H20?
Answers
Answer: There are therefore 6.02 × 1023 water molecules in a mole of water molecules.
Explanation: Hope this helps
C7H6O3+ C4H6O3=C9H8O4+H20
- Balance the above equation.
Answers
Answer:
2C7H6O3 + C4H6O3 - - - > 2C9H8O4 + H2O
Explanation:
Reactants:
2C7H6O3 + C4H6O3
C = 18
H = 18
O = 9
Products:
2C9H8O4 + H2O
C = 18
H = 18
O = 9
True or false: All molecules or atoms in a substance have the same amount of kinetic energy at a given temperature?
Answers
Answer:
1
False. if they all moved together at any time then it would be a million things withinthe human species all at once at a high rate
Brainliest to right answer
Answers
Crust
Upper mantle
Lower mantle
Outer core
Inner core.
What will a balloon do to a cloth if its negatively charged
Answers
Answer:
This involves negatively charged particles (electrons) jumping to positively charged objects. When you rub the balloons against the fabric they become negatively charged. They take some of the electrons from the fabric and leave them positively charged.
Explanation:
Negative charges attract to positive charges. If a balloon is not rubbed with the wool cloth, it has an equal amount of negative to positive charges, so it will attract to a rubbed balloon. When both balloons are rubbed with the wool cloth, the both receive negative charges, so they will repel each other.
Question: why do astronauts wear organge suits vs white suits
Full answer please..
Answers
convert 0.881 mol N2 at STP to volume in liters
Answers
Answer:
19.7 L N2
Explanation:
The volume of the nitrogen gas at STP is equal to 19.72 L.
What is the ideal gas equation?The ideal gas equation can be defined as an equation that describes the behavior of an ideal gas. This equation gives by the product of the volume and pressure is equal to the product of the moles of gas, gas constant, and absolute temperature.
The mathematical form of the ideal gas law is given as follows:
PV = nRT
Where n is the moles, V is the volume, R is the universal gas constant and P is the pressure.
The temperature of the N₂ , T = 0° C = 273 K
The pressure of the gas at STP, P = 1 atm
The value of the universal gas constant, R = 0.082 atm L/K mol
The number of moles of N₂, n = 0.881 mol
Fill in the values n, R, P, and T in the equation, and we get:
1 × V = 0.881 × 0.082 × 273
V = 19.72 L
Learn more about ideal gas equation, here:
brainly.com/question/3637553
#SPJ2
need help to solve it
Answers
Answer:
Divide by the molar mass to get moles
Explanation:
How many grams of silver nitrate are required to produce 3.00 g of silver phosphate?
Answers
Answer:
105.3
Explanation:
Pleade help me! (If you guys unfortunately I am going to report you) Thank you
Answers
Sorry if I’m wrong
What is the pH of a 2.0 × 10-4 M HCl solution?
Answers
Answer:
pH = 3.69
Explanation:
HCl is a strong acid. It will disscociates completely into dissoluted ions such that,
[tex][H^+]=2\times 10^{-4}\ M[/tex]
The formula for pH is given by :
[tex]pH=-log[H^+]\\\\pH=-log(2\times 10^{-4})\\\\pH=3.69[/tex]
So, the pH of the solution is 3.69.
The answer to your question is 4.