Answers
Answer:
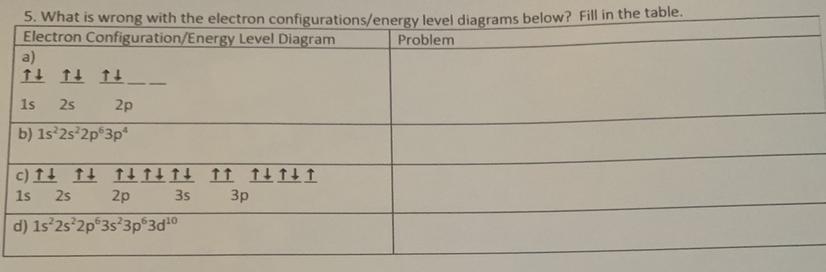
a.) Instead of configuring all up before some down, all of the configurations were placed as up and down, leaving two spots empty in the 2p sublevel.
b.) There is a missing s sublevel for row 3.
c.) There are two up arrows in one of the lines.
d.) When you get to the "d" section you must subtract the number you're using by 1. So, it's supposed to be 2d to the power of 10.
Related Questions
How many moles are in 670 g of gold (|||) chloride
Answers
There are 2.208 moles of gold (III) chloride in 670 g.
To determine the number of moles in 670 g of gold (III) chloride, we need to first calculate the molar mass of gold (III) chloride, which is AuCl3.
The atomic mass of gold is 196.97 g/mol and the atomic mass of chlorine is 35.45 g/mol. Since there are three chlorine atoms in each molecule of gold (III) chloride, we multiply the atomic mass of chlorine by 3:
35.45 g/mol x 3 = 106.35 g/mol
Adding the atomic masses of gold and chlorine together gives us the molar mass of gold (III) chloride:
196.97 g/mol + 106.35 g/mol = 303.32 g/mol
Now, we can use this molar mass to convert 670 g of gold (III) chloride into moles:
670 g / 303.32 g/mol = 2.208 moles
Therefore, there are 2.208 moles of gold (III) chloride in 670 g.
Know more about molar mass here:
https://brainly.com/question/837939
#SPJ11
A balloon is rubbed against a wall. The picture on the left shows the balloon and the wall before rubbing. The picture on the right shows the balloon and the wall after rubbing.
What happened when the balloon was rubbed against the wall? (5.b)
2. A balloon is rubbed against a wall. The picture on the left shows the balloon and the wall before rubbing. The picture on the right shows the balloon and the wall after rubbing.
What happened when the balloon was rubbed against the wall?
A. Electrons were transferred from the wall to the balloon.
B. Protons were transferred from the wall to the balloon.
C. Electrons were transferred from the balloon to the wall.
D. Protons were transferred from the balloon to the wall.
Answers
Answer: The answer should be A
Explanation:
each of the following can act as both an brönsted acid and a brönsted base except:
(A) HCO3
(B) NH4+
(C) HS
(D) H2PO4
Answers
The answer is (C) HS.
Each of the other options can donate a proton (act as a Brönsted acid) in certain conditions and accept a proton (act as a Brönsted base) in other conditions. However, HS is only capable of acting as a Brönsted base and accepting a proton, but it cannot donate a proton and act as a Brönsted acid.
Out of the given options, the one that cannot act as both an acid and a base is (C) HS. This is because HS can only act as a brönsted acid by donating a proton to a brönsted base, but it cannot act as a brönsted base by accepting a proton from a brönsted acid. This is because it lacks a lone pair of electrons on the sulfur atom, which is necessary for accepting a proton.
On the other hand, [tex]HCO_{3}[/tex] ,[tex]NH_{4}[/tex]+, and [tex]H_{2}[/tex][tex]O_{4}[/tex]P can all act as both brönsted acids and bases depending on the reaction conditions.
Learn more about Brønsted acid here:
https://brainly.com/question/24065957
#SPJ11
(B) NH4⁺, cannot act as both a Brønsted acid and a Brønsted base.
What is Bronsted Acid-Base pairs?
A Brønsted acid is a species that can donate a proton (H⁺), while a Brønsted base is a species that can accept a proton (H⁺).
(A) HCO3⁻ can act as an acid by donating a proton to form CO3²⁻ or as a base by accepting a proton to form [tex]H_{2}CO_{3}[/tex].
(C) HS⁻ can act as an acid by donating a proton to form S²⁻ or as a base by accepting a proton to form [tex]H_{2}S[/tex].
(D) H2PO4⁻ can act as an acid by donating a proton to form HPO4²⁻ or as a base by accepting a proton to form [tex]H_{3}PO_{4}[/tex].
However,
(B) NH4⁺ can only act as a Brønsted acid by donating a proton to form [tex]NH_{3}[/tex] but cannot act as a Brønsted base since it has no lone pair of electrons to accept a proton.
To know more about Bronsted Theory:
https://brainly.com/question/148529
#SPJ11
why do you think scientists chose the top of mauna loa, hawaii, as the best place to measure atmospheric co2 concentrations?
Answers
The scientists chose the top of Mauna Loa, Hawaii, is the best place to measure the atmospheric CO₂ concentrations is because to measure the CO₂ in the air masses which could be representative the Northern Hemisphere, and the globe.
To measure the CO₂ in the air masses which could be representative the Northern Hemisphere, and the globe. The rise in level of the atmospheric CO₂ concentrations and this resulted in the global warming and the climate change.
The climate change is the serious consequences, it also including the rising sea levels, it will be more frequent and the severe weather events, it will increased the risk of the droughts and the wildfires.
To learn more about atmospheric CO₂ here
https://brainly.com/question/31036310
#SPJ4
what do you suspect is the solid or oil that was not soluble in hexanes after synthesizing the adipoyl chloride?
Answers
Without more information about the synthesis process and the specific substances used, it's difficult to say exactly what the solid or oil that was not soluble in hexanes might be. However, there are a few possibilities to consider.
One possibility is that the solid or oil is an impurity that was introduced during the synthesis process. For example, it could be a side product or a reactant that did not fully react with the adipoyl chloride. In this case, the substance may not be soluble in hexanes because it has different chemical properties than the desired product.
Another possibility is that the substance is a byproduct of the reaction between the adipoyl chloride and another substance, such as a solvent or a catalyst. In this case, the substance may not be soluble in hexanes because it has a different chemical structure than the desired product and is not compatible with hexanes.
Alternatively, it's possible that the solid or oil is a form of the adipoyl chloride itself. For example, if the adipoyl chloride was not fully purified or if it was synthesized using impure starting materials, it could contain other compounds that are not soluble in hexanes.
Overall, without more information about the synthesis process and the specific substances used, it's difficult to determine the exact nature of the solid or oil that was not soluble in hexanes. Further analysis, such as chromatography or spectroscopy, may be necessary to identify the substance and determine its origin.
one of the techniques used in this experiment was that of crystallization. when cooling a solution in the process of crystallization, why would an ice bath be preferable over cold water or ice alone? none of the answers shown are correct. ice is too cold and will freeze any solution. cold water would dilute the solution making it impossible for crystals to form. a mixture of ice and water will keep the temperature above freezing and will contact the entire portion of the container immersed in the ice/water mixture.
Answers
When conducting a crystallization process, it is important to cool the solution at a slow and controlled rate to encourage crystal formation.
An ice bath is preferable over cold water or ice alone because it can maintain a consistent low temperature without causing the solution to freeze solid. Ice alone is too cold and can cause the solution to freeze rapidly, preventing the formation of crystals. Cold water, on the other hand, is not able to maintain a consistent low temperature as the heat from the solution will quickly dissipate into the surrounding water, resulting in a slower cooling rate.
An ice bath, which is a mixture of ice and water, provides a more stable and uniform cooling environment for the solution, allowing for the crystals to form at a slower rate. Additionally, an ice bath can contact the entire portion of the container immersed in the mixture, ensuring that the solution is evenly cooled. Overall, an ice bath is the preferred method for cooling a solution during the process of crystallization.
know more about crystallization process here
https://brainly.com/question/29662937#
#SPJ11
complete question is:-
one of the techniques used in this experiment was that of crystallization. when cooling a solution in the process of crystallization, why would an ice bath be preferable over cold water or ice alone? none of the answers shown are correct. ice is too cold and will freeze any solution. cold water would dilute the solution making it impossible for crystals to form. a mixture of ice and water will keep the temperature above freezing and will contact the entire portion of the container immersed in the ice/water mixture. EXPLAIN.
Help what's the answers?
Answers
The number of moles of bromine trifluoride needed to produce 23.2 L of fluorine gas according to the reaction would be 0.339 moles.
Stoichiometric problemsThe balanced equation for the reaction is:
BrF3 → Br + 3F2
From the equation, we can see that 1 mole of BrF3 produces 3 moles of F2. Therefore, to calculate the number of moles of BrF3 needed to produce 23.2 L of F2 at 0°C and 1 atm, we need to use the ideal gas law:
PV = nRT
where P is the pressure, V is the volume, n is the number of moles, R is the gas constant, and T is the temperature.
We can rearrange the ideal gas law to solve for n:
n = PV/RT
At 0°C (273 K) and 1 atm, the value of R is 0.08206 L·atm/mol·K. Substituting the values given, we get:
n = (1 atm) × (23.2 L) / (0.08206 L·atm/mol·K × 273 K)
n = 1.017 mol F2
Since 1 mole of BrF3 produces 3 moles of F2, we need 1/3 as many moles of BrF3:
n(BrF3) = 1.017 mol F2 × (1 mol BrF3 / 3 mol F2)
n(BrF3) = 0.339 mol BrF3
Therefore, 0.339 moles of BrF3 are needed to produce 23.2 L of F2 at 0°C and 1 atm.
More on stoichiometric problems can be found here: https://brainly.com/question/14465605
#SPJ1
154.42g of oxygen gas (O2) react with an excess of ethane (C2H6) produces how many moles of water vapor (H2O)?
Answers
2.77 moles of water vapour (H2O) are created when 154.42 g of oxygen gas (O2) reacts with an excess of ethane (C2H6).
Calculation-
In order to create water vapour [tex](H_2O)[/tex], ethane [tex](C_2H_6)[/tex]and oxygen gas (O2) must be burned. The chemical equation for this reaction is:
[tex]C_2H_6 + 7O_2 -- > 4H_2O + 6CO_2[/tex]
We may deduce from the equation that when 1 mole of ethane (C2H6) interacts with 7 moles of oxygen gas (O2), 4 moles of water vapour (H2O) are created.
We must utilise its molar mass to translate the 154.42 g of oxygen gas (O2) consumed into moles. 32 g/mol (16 g/mol for each oxygen atom multiplied by two for O2) is the molar mass of oxygen gas.
Moles of oxygen gas (O2) = Mass of oxygen gas (O2) / Molar mass of oxygen gas (O2)
Moles of oxygen gas (O2) = 154.42 g / 32 g/mol
Moles of oxygen gas (O2) = 4.83 mol (rounded to two decimal places)
The balanced equation's stoichiometry predicts that 7 moles of oxygen gas [tex](O_2)[/tex]and 4 moles of water vapour [tex](H_2O)[/tex] will react. We can thus calculate the moles of water vapour [tex](H_2O)[/tex] created using the stoichiometric principle.
Moles of water vapor [tex](H_2O)[/tex] = Moles of oxygen gas [tex](O_2)[/tex] × (4 moles of [tex]H_2O[/tex] / 7 moles of O2)
Moles of water vapor [tex](H_2O)[/tex] = 4.83 mol × (4/7)
Moles of water vapour[tex](H_2O)[/tex] = 2.77 mol (rounded to two decimal places)
to know more about water vapour here:
brainly.com/question/20899075
#SPJ1
what is the ph of a solution prepared by mizing 100ml of 0.020m ba(oh)2 with 50ml of 0.400m of koh? assume that the volumes are addative
Answers
The pH of the solution is approximately 12.73.
First, we need to find the moles of each solution:
moles of Ba(OH)2 = 0.020 mol/L x 0.100 L = 0.002 mol
moles of KOH = 0.400 mol/L x 0.050 L = 0.020 mol
Next, we need to find the total volume of the solution:
Vtotal = 100 mL + 50 mL = 150 mL = 0.150 L
Now, we can find the total concentration of OH- ions:
[OH-] = moles of Ba(OH)2 + moles of KOH / Vtotal
[OH-] = (0.002 mol + 0.020 mol) / 0.150 L = 0.187 mol/L
Finally, we can find the pH of the solution using the following formula:
pH = 14 - log([OH-])
pH = 14 - log(0.187) = 12.73
Therefore, the pH of the solution is approximately 12.73.
Learn more about the moles
https://brainly.com/question/18265914
#SPJ4
the most common constituent of gas in the disk of the milky way galaxy is ________.
Answers
The most common constituent of gas in the disk of the Milky Way galaxy is hydrogen gas.
Hydrogen gas is the most abundant element in the Milky Way galaxy, making up around 75% of its elemental mass. This is why hydrogen is often used as a tracer for studying the structure and dynamics of galaxies. The gas in the disk of the Milky Way is mostly composed of atomic hydrogen (H I) and molecular hydrogen (H2), with smaller amounts of other elements like helium and carbon. Studying the distribution and properties of this gas can provide insight into the formation and evolution of the Milky Way.
learn more about gas here
https://brainly.com/question/28549254
#SPJ11
The most common constituent of gas in the disk of the Milky Way galaxy is hydrogen gas.
The most common constituent of gas in the disk of the Milky Way galaxy is hydrogen. Hydrogen is the most abundant element in the universe and makes up the majority of the gas in the disk of the Milky Way galaxy, with its presence primarily in the form of atomic and molecular hydrogen. It is often found in the form of molecular hydrogen ([tex]H_{2}[/tex]) in interstellar clouds, which are regions of gas and dust where stars are formed. Other common constituents of gas in the Milky Way galaxy's disk include helium (He), carbon (C), oxygen (O), nitrogen (N), and trace amounts of other elements.
To know more about Milky Way:
https://brainly.com/question/30417276
#SPJ11
2. calculate the ph of a solution prepared by mixing 25.0 ml of 0.60 m hc2h3o2 and 15.0 ml of 0.60 m naoh?
Answers
The Ph of a solution is 8.46
The reaction is:
[tex]HC_2H_3O+2 + NaOH - > NaC_2H_3O_2 + H_2O[/tex]
This is a neutralization reaction, where the acid HC2H3O2 reacts with the base NaOH to form the salt NaC2H3O2 and water.
Next, we need to calculate the amount of each reagent used in the reaction. To do this, we use the equation:
Molarity (M) = moles (mol) / volume (L)
For [tex]HC_2H_3O_2[/tex]:
M = 0.60 M
Volume = 25.0 ml = 0.025 L
moles = M x volume = 0.60 M x 0.025 L = 0.015 mol
For NaOH:
M = 0.60 M
Volume = 15.0 ml = 0.015 L
moles = M x volume = 0.60 M x 0.015 L = 0.009 mol
Since the reaction is a 1:1 stoichiometry, we can see that 0.009 mol of NaOH is enough to react with all the HC2H3O2 in the solution, leaving some excess NaOH. Therefore, we need to calculate the concentration of the remaining NaOH in the solution:
moles of NaOH remaining = moles of NaOH added - moles of HC2H3O2 reacted
= 0.009 mol - 0.015 mol = -0.006 mol (negative sign indicates there is no excess NaOH remaining)
To calculate the concentration of the NaOH that reacted, we need to subtract the moles of NaOH remaining from the total moles of NaOH added:
moles of NaOH reacted = moles of NaOH added - moles of NaOH remaining
= 0.009 mol - (-0.006 mol) = 0.015 mol
The volume of the final solution is:
Total volume = volume of HC2H3O2 + volume of NaOH
= 25.0 ml + 15.0 ml = 0.040 L
The concentration of NaC2H3O2 in the final solution is:
Molarity (M) = moles / volume
M = 0.015 mol / 0.040 L = 0.375 M
Now, we need to calculate the pH of the solution. NaC2H3O2 is the conjugate base of HC2H3O2, which means it will hydrolyze in water to form OH- ions:
NaC2H3O2 + H2O ⇌ NaOH + HC2H3O2
The equilibrium constant for this reaction is called the base dissociation constant (Kb) and is given by:
Kb = [NaOH] [HC2H3O2] / [NaC2H3O2]
We can use the relationship:
Kw = Ka x Kb
Where Kw is the ion product constant for water, which is 1.0 x 10^-14 at 25°C, and Ka is the acid dissociation constant for HC2H3O2, which is 1.8 x 10^-5 at 25°C.
Rearranging the equation, we get:
Kb = Kw / Ka = 1.0 x 10^-14 / 1.8 x 10^-5 = 5.6 x 10^-10
Next, we need to calculate the concentration of HC2H3O2 and NaOH that are present in the solution after hydrolysis. Since NaC2H3O2 is a strong electrolyte,
it will completely dissociate in water to form Na+ and C2H3O2- ions. Therefore, the concentration of Na+ ions will be equal to the concentration of NaC2H3O2, which is 0.375 M.
The concentration of OH- ions can be calculated from the Kb expression:
Kb = [OH-]^2 / [HC_2H_3O_2]
[OH-]^2 = Kb x [[tex]HC_2H_3O_2[/tex]] = 5.6 x 10^-10 x 0.015 M = 8.4 x 10^-12
[OH-] = 2.9 x 10^-6 M
The pH of the solution can be calculated from the relationship:
pH + pOH = 14
pOH = -log [OH-] = -log (2.9 x 10^-6) = 5.54
pH = 14 - pOH = 14 - 5.54 = 8.46
To learn more about : Ph
https://brainly.com/question/172153
#SPJ11
problem 9.34 the cis and trans isomers of 2,3-dimethyloxirane both react with to give butane-2,3-diol. one stereoisomer gives a single achiral product, and one gives two chiral enantiomers. which epoxide gives one product and which gives two?
Answers
The reaction of both cis and trans isomers of 2,3-dimethyloxirane with HBr gives butane-2,3-diol. However, one of these stereoisomers gives a single achiral product, while the other gives two chiral enantiomers.
The reaction of 2,3-dimethyloxirane with itself is an example of an intramolecular nucleophilic substitution reaction.
The cis isomer of 2,3-dimethyloxirane has a plane of symmetry and is therefore an achiral molecule. When it reacts with itself, it will only form a single product nucleophilic substitution reaction.
The trans isomer of 2,3-dimethyloxirane is a chiral molecule and does not have a plane of symmetry. When it reacts with itself, it will form two enantiomers of the product, one being the mirror image of the other.
Learn more about 2,3-dimethyloxirane
https://brainly.com/question/15181918
#SPJ4
Question:
The Volume (V) of gas varies
directly as the temperature (T) and
inversely as the pressure (P). If the
volume is 225 cm³ when the
temperature is 300 K and the
pressure is 100 N/cm², what is the
volume when the temperature
drops to 270 K and the pressure is
150 N/cm²?
Answers
The volume of the gas when the temperature drops to 270 K and the pressure is 150 N/cm², is 135 cm³
How do I determine the volume of the gas?
The following data were obtained from the question.
Initial volume of gas (V₁) = 225 cm³Initial temperature of gas (T₁) = 300 KInitial pressure of gas (P₁) = 100 N/cm²New temperature (T₂) = 270 KNew pressure (P₂) = 150 N/cm²New volume of gas (V₂) = ?The new volume of the gas can be obtained by using the combined gas equation as illustrated below:
P₁V₁ / T₁ = P₂V₂ / T₂
(100 × 225) / 300 = (150 × V₂) / 270
Cross multiply
300 × 150 × V₂ = 100 × 225 × 270
Divide both side by (300 × 150)
V₂ = (100 × 225 × 270) / (300 × 150)
V₂ = 135 cm³
Thus, the volume of the gas is 135 cm³
Learn more about volume:
https://brainly.com/question/14560487
#SPJ1
Lab: Relative and Absolute Dating Lab Report What is the purpose of the lab?
Answers
The goal of a Relative and Absolute Dating Lab Report is to discover and utilize the concepts of relative and absolute dating methods for determining the age of geological materials like rocks and fossils.
What is the point of absolute dating?Geologists frequently need to know the age of the material they find. They use absolute dating methods, also known as numerical dating, to give rocks an exact date, or date range, in years. This is distinct from relative dating, which only places geological events in chronological order.
What exactly is the concept of relative dating?Relative dating is the process of determining whether one rock or geologic event is older or younger than another without knowing their exact ages that is, how many years ago the object was formed.
Where can the relative dating method be used?Relative dating is used to order geological events and the rocks they leave behind. Stratigraphy is the process of reading the order. Relative dating does not yield precise numerical dates for the rocks.
To know more about the Lab visit:
https://brainly.com/question/29869193
#SPJ1
what atomic or hybrid orbitals make up the sigma bond between b and f in tetrafluoroborate ion, bf4-?
Answers
The sigma bond between b and f in tetrafluoroborate ion, bf4-, is formed by the overlap of the atomic orbitals of boron and fluorine. Specifically, each of which contributes one p orbital to form a sp3-p sigma bond.
In the tetrafluoroborate ion (BF4-), the bond between boron (B) and fluorine (F) is a sigma (σ) bond. The σ bond is formed by the overlap of atomic or hybrid orbitals.Boron in BF4- is sp3 hybridized, which means that it has four hybrid orbitals that are involved in bonding. Three of these hybrid orbitals are involved in bonding with three of the fluorine atoms, while the fourth hybrid orbital is used to form the σ bond with the fourth fluorine atom.Fluorine is a halogen and has the electron configuration of 1s2 2s2 2p5. In BF4-, each of the fluorine atoms is also involved in the formation of the σ bond with boron. Fluorine has three unpaired electrons in its 2p orbitals that can form a σ bond by overlapping with the sp3 hybrid orbital of boron.Therefore, the σ bond between boron and fluorine in BF4- is formed by the overlap of the sp3 hybrid orbital of boron and the 2p orbital of the fluorine atom.
Learn more about fluorine here
https://brainly.com/question/1940697
#SPJ11
A closed system is one which no matter can enter or exit. True or false
Answers
False. In a closed system, matter can not enter or exit that is there is no change in the matter of the system.
Three types of systems exist in nature:
1. Open System: In this system, the matter can interact with the surroundings or matter can enter or exit the system from the surrounding. Similarly, the energy of the system also interacts with its surroundings and can be lost or gained.
For example oceans etc.
2. Closed system: In this system, the matter is unable to interact with the surroundings that are matter can't exit or enter the system. While the energy of the system is able to interact with the surroundings.
For example Earth etc
3. Isolated system: In this system, both matter and energy are unable to interact with the surrounding. There is no exchange between matter and the energy of surroundings.
For example thermos-teel bottles etc.
Learn more about Open Systems:
https://brainly.com/question/28891854
#SPJ4
which term is defined as a pollutant that is formed by a chemical reaction between a primary pollutant and another compound in the atmosphere (either natural or human-made)
Answers
The term defined as a pollutant that is formed by a chemical reaction between a primary pollutant and another compound in the atmosphere (either natural or human-made) is "secondary pollutant".
Primary pollutants are directly emitted into the atmosphere from sources such as cars, factories, and power plants. Examples of primary pollutants include carbon monoxide (CO), sulfur dioxide (SO₂), and nitrogen oxides (NOₓ).
Secondary pollutants, on the other hand, are not directly emitted into the atmosphere, but are formed through chemical reactions between primary pollutants and other compounds in the atmosphere. Examples of secondary pollutants include ground-level ozone (O₃), which is formed through the reaction of NOₓ and volatile organic compounds (VOCs), and acid rain, which is formed through the reaction of SO₂ and NOₓ with water, oxygen, and other chemicals in the atmosphere.
The formation of secondary pollutants is often dependent on factors such as temperature, sunlight, and the presence of other chemicals in the atmosphere. Secondary pollutants can be just as harmful to human health and the environment as primary pollutants, and are an important consideration in air pollution control strategies.
To learn more about pollutants, here
https://brainly.com/question/28519286
#SPJ4
how many moles of naf must be dissolved in 1.00 liter of a saturated solution of pbf2 at 25˚c to reduce the [pb2 ] to 1 x 10–6 molar? (ksp pbf2 at 25˚c = 4.0 x 10–8)
Answers
The moles of NaF that must be dissolved in 1.00 liter of a saturated solution of PbF₂ at 25˚C to reduce the [Pb²⁺] to 1 x 10⁻⁶ molar is 2.0 x 10⁻⁵.
The solubility product expression for PbF₂ is given by:
Ksp = [Pb²⁻][F-]²At equilibrium, the product of the ion concentrations must be equal to the solubility product constant. We are given that the [Pb²⁺] in the saturated solution is 1 x 10⁻⁶ M. Therefore, we can use the Ksp expression to calculate the concentration of F- in the solution:
Ksp = [Pb²⁺][F⁻]²4.0 x 10⁻⁸ = (1 x 10⁻⁶)([F⁻]²)[F⁻]² = 4.0 x 10⁻²[F⁻] = 2.0 x 10⁻¹Now, we can calculate the amount of NaF needed to reduce the [F⁻] concentration to 2.0 x 10⁻¹ M. Since NaF is a 1:1 electrolyte, the concentration of F- will be equal to the concentration of NaF added.
Number of moles of NaF = (2.0 x 10⁻¹) mol/L x 1.00 L = 2.0 x 10⁻¹ molesHowever, we need to dissolve this amount of NaF in a saturated solution of PbF₂. Therefore, we need to check that the amount of NaF we added will not exceed the maximum amount that can dissolve in the solution at 25˚C.
To learn more about solubility, here
https://brainly.com/question/29661360
#SPJ4
2-thiosubstituted chlorocyclohexanes can undergo an sn2 reaction with intramolecular catalysis. which stereoisomer is the most reactive?
Answers
The axial stereoisomer is the most reactive in this type of reaction.
In an SN2 reaction with intramolecular catalysis, the most reactive stereoisomer is the one with an axial thioether group.
This is because in the axial position, the thioether group is closer to the leaving group (chlorine), allowing for more efficient overlap of their orbitals and a lower energy transition state.
The equatorial thioether group is farther away from the leaving group, resulting in a higher energy transition state and a slower reaction. Therefore, the axial stereoisomer is the most reactive in this type of reaction.
Learn more about stereoisomer
https://brainly.com/question/31147524
#SPJ4
does this suggest that your reaction worked? use three key signals to justify your answer 1-methoxy-2-chloro-4-nitrobenzene
Answers
Yes, the reaction worked. Three key signals that suggest the reaction worked include the appearance of the product, the presence of the expected starting material, and the absence of any other byproducts.
The product, 1-methoxy-2-chloro-4-nitrobenzene, can be identified by its distinct color, smell, and boiling point. Additionally, if the expected starting material is present, then it shows that the reaction has taken place.
Lastly, the absence of any other byproducts such as unreacted starting material implies that the reaction was successful. All together, all three signals indicate that the reaction worked.
Know more about Three key signals here
https://brainly.com/question/31114075#
#SPJ11
naoh is a hygroscopic solid, which means that it can absorb water from its surroundings, therefore it is important to
Answers
As a result, it is important to store NaOH in a dry and cool place, away from any sources of moisture or water.
NaOH, also known as sodium hydroxide, is a highly hygroscopic solid. This means that it can easily absorb moisture from its surroundings, including the air. When NaOH absorbs water, it can become more corrosive and potentially dangerous.
This is why it is also important to handle NaOH with care and wear appropriate protective gear, such as gloves and goggles. Additionally, any spills or leaks should be cleaned up immediately and properly disposed of according to local regulations.
By following these precautions, NaOH can be safely used in a variety of applications, including in the production of soap, paper, and textiles.
To learn more about : water
https://brainly.com/question/19491767
#SPJ11
why would it be necessary to slowly add the sulfuric acid to the p-cresol/acetic acid mixture in the test tube? simply to be sure the correct volumes are used. the reaction is exothermic which may boil and splatter the acidic solution out of the test tube. since the density of sulfuric acid is less than that for acetic acid, it requires a slower reaction time. the reaction is endothermic and the solution may solidify if the sulfuric acid is added too quickly.
Answers
The correct answer is option D. All of the above. It is necessary to slowly add the sulfuric acid to the p-cresol/acetic acid mixture in the test tube to prevent any accidents or injuries.
If sulfuric acid is added too soon, the solution may boil and the acid will spew out of the test tube, perhaps resulting in burns.
Sulfuric acid is also an endothermic reaction, which means it takes energy from its surroundings and has the potential to crystallise or cause the solution to harden.
Last but not least, adding the sulfuric acid gradually enables more precise measurement of the supplied sulfuric acid volume.
It is crucial to gradually add the sulfuric acid to the test tube mixture of p-cresol and acetic acid as a result of all these considerations.
Complete Question:
Why would it be necessary to slowly add the sulfuric acid to the p-cresol/acetic acid mixture in the test tube?
Options:
A. To ensure accurate measurement of the volume of sulfuric acid added.
B. To prevent the solution from boiling and splattering the acidic solution out of the test tube.
C. To prevent the endothermic reaction from solidifying the solution.
D. All of the above.
To learn more about sulfuric acid visit:
https://brainly.com/question/10220770
#SPJ4
energetic molecules such as nadh and atp are often reactants of ____________ reactions.
Answers
Energetic molecules such as NADH and ATP are often reactants of exergonic reactions.
Exergonic reactions are those that discharge energy and have a harmful Gibbs-free energy change. In these reactions, the reactants have more free energy than the products, so the excess energy is cast in the state of heat. An exergonic reaction is a chemical reaction where the shift in the free energy is negative.
Energetic molecules like NADH and ATP store energy in their chemical adhesives, which can be emitted in exergonic reactions to drive endergonic responses that need energy input. Therefore, they are usually employed as reactants in exergonic reactions.
To learn more about Exergonic reactions
https://brainly.com/question/30800156
#SPJ4
How many molecules of carbon dioxide gas, CO2, are found in 0.125 moles
Answers
There are 7.52 x 10^22 molecules of carbon dioxide gas, CO2, in 0.125 moles.
The number of molecules in a given number of moles can be calculated using Avogadro’s number, which is approximately 6.022 x 10^23. This number represents the number of particles (atoms or molecules) in one mole of a substance.
To calculate the number of molecules in 0.125 moles of CO2, we can multiply the number of moles by Avogadro’s number: 0.125 moles x (6.022 x 10^23 molecules/mole) = 7.52 x 10^22 molecules.
Avogadro’s number is a fundamental constant in chemistry and is used in many calculations involving moles and molar mass.
To learn more about carbon dioxide,
brainly.com/question/3049557
Write the expression for the equilibrium constant for each of the following reaction:
2Fe2O3(s)+3C(s)⇌4Fe(s)+3CO2(g)
A) Kc=[CO2]3
B) Kc=[Fe]4[CO2]3[Fe2O3]2[C]3
C) Kc=[Fe2O3]2[C]3[Fe]4[CO2]3
D) Kc=2[Fe2O3]3[C]4[Fe]3[CO2]
Answers
The correct expression for the equilibrium constant (Kc) for the reaction:
[tex]2Fe2O3(s) + 3C(s) ⇌ 4Fe(s) + 3CO2(g)[/tex] is: [tex]Kc=[Fe]4[CO2]3/[Fe2O3]2[C]3[/tex]
The equilibrium constant expression for the given reaction, [tex]2Fe2O3(s) + 3C(s) ⇌ 4Fe(s) + 3CO2(g)[/tex] is written as the ratio of the product concentrations raised to their respective coefficients divided by the reactant concentrations raised to their respective coefficients.
The ratio of the equilibrium concentrations of the products to the concentrations of the reactants raised to their respective powers to match the coefficients in the equilibrium equation at equilibrium is K, according to the law of mass action. The equilibrium constant expression is known as the ratio, a condition where there is a balance between opposing and static forces.
In this case, it would be:
[tex]Kc = ([Fe]^4[CO2]^3)/([Fe2O3]^2[C]^3)[/tex]
learn more about equilibrium constants here:
https://brainly.com/question/3159758
#SPJ11
The correct expression for the equilibrium constant for the given reaction is:
C) Kc=[Fe2O3]2[C]3[Fe]4[CO2]3
The equilibrium constant (Kc) for a chemical reaction is written using the concentrations of the species involved in the reaction. Here's the general format for writing the equilibrium constant expression:
For the generic reaction:
aA + bB ⇌ cC + dD
The equilibrium constant (Kc) expression would be: Kc = [C]^c [D]^d / [A]^a [B]^b
where [A], [B], [C], and [D] represent the concentrations of the respective species at equilibrium, and a, b, c, and d are the stoichiometric coefficients of the species in the balanced chemical equation.
To know more about Equilibrium Constant:
https://brainly.com/question/29117627
#SPJ11
The presence of an alcohol group (-OH), __________ the ΔT value of a molecule compared to the presence of a methyl group (-CH3).
A. increases
B. decreases
C. stays the same
Answers
The presence of an alcohol group (-OH) in a molecule, compared to the presence of a methyl group (-CH3), increases the ΔT value of a molecule.
The presence of an alcohol group (-OH) leads to the formation of hydrogen bonds, which are stronger than the van der Waals forces present in molecules with a methyl group (-CH3). As a result, more energy is required to break these hydrogen bonds, leading to a higher ΔT value (a greater change in temperature during phase transitions).
Therefore the correct answer is A. increases.
To learn more about alcohol, refer:-
https://brainly.com/question/16975086
#SPJ11
consider a reaction between two gaseous reactants (4 mol of a and 4 mol of b) in the closed flasks shown below. assume that the two reactions are both at room temperature. which reaction will occur faster?
Answers
Answer:
....................................................
Factors such as pressure, volume, and the presence of catalysts can affect the rate of the reaction.
Figure out the reaction between two gaseous reactants?The two gaseous reactants (4 mol of A and 4 mol of B) in the closed flasks shown below will occur faster, I would need more information about the specific conditions in each flask. Factors such as pressure, volume, and the presence of catalysts can affect the rate of the reaction.
If you could provide more details about the flasks and the conditions, I would be happy to help you determine which reaction will occur faster.
Learn more about Gaseous reactants
brainly.com/question/28297794
#SPJ11
If I have an unknown quantity of gas at a pressure of 1.35 atm, a volume of 25 liters, and a temperature of 300. K, how many moles of gas do I have?
Answers
Answer:
We can use the ideal gas law to solve for the number of moles of gas:
PV = nRT
where P is pressure, V is volume, n is the number of moles, R is the gas constant, and T is temperature in Kelvin.
Plugging in the given values:
(1.35 atm)(25 L) = n(0.0821 L·atm/mol·K)(300 K)
n = (1.35 atm)(25 L) / (0.0821 L·atm/mol·K)(300 K)
n = 1.29 mol
Therefore, there are 1.29 moles of gas in the container.
What volume of chlorine gas at 46.0◦C and
1.60 atm is needed to react completely with
5.20 g of sodium to form NaCl?
Answers
The volume of chlorine gas at 46.0°C and 1.60 atm that is needed to react completely with 5.20 g of sodium to form NaCl is 1.85 L
How do i determine the volume of chlorine gas needed?We'll begin by obtaining the mole of 5.20 g of sodium. Details below:
Mass of Na = 5.20 gMolar mass of Na = 23 g/mol Mole of Na =?Mole = mass / molar mass
Mole of Na = 5.20 / 23
Mole of Na = 0.226 mole
Next, we shall determine the mole of chlorine gas needed. Details below:
2Na + Cl₂ -> 2NaCl
From the balanced equation above,
2 moles of Na reacted with 1 mole of Cl₂
Therefore,
0.226 mole of Na will react with = (0.226 × 1) / 2 = 0.113 mole of Cl₂
Finally, we shall determine the volume of chlorine gas, Cl₂ needed. This is shown below:
Temperature (T) = = 46 °C = 46 + 273 = 319 KPressure (P) = 1.60 atmGas constant (R) = 0.0821 atm.L/molKNumber of mole (n) = 0.113 moleVolume of chlorine gas, Cl₂ (V) =?PV = nRT
1.6 × V = 0.113 × 0.0821 × 319
Divide both sides by 1.6
V = (0.113 × 0.0821 × 319) / 1.6
V = 1.85 L
Thus, the volume of chlorine gas, Cl₂ needed is 1.85 L
Learn more about volume:
https://brainly.com/question/21838343
#SPJ1
N2O4 ⇌ 2NO2
for the following reaction at 373 K, Kc = 0.36. If initial concentration of N2O4 is 0.1 mol dm^-3, what is the equilibrium concentration of NO2? (Is there a way to solve this without using quadratics?)
Answers
Okay, let's solve this step-by-step without using quadratics:
1) The equilibrium constant Kc = 0.36 means the equilibrium lies to the left. So there will be more N2O4 than NO2 at equilibrium.
2) The initial concentration of N2O4 is 0.1 mol dm^-3. Let's call this [N2O4]initial.
3) At equilibrium, the concentrations of N2O4 and NO2 will be [N2O4]equil and [NO2]equil respectively.
4) We know the equilibrium constant expression for this reaction is:
Kc = ([NO2]equil)^2 / [N2O4]equil
5) Setting this equal to 0.36 and plugging in 0.1 for [N2O4]initial, we get:
0.36 = ([NO2]equil)^2 / (0.1 - [NO2]equil)
6) Simplifying, we get:
0.036 = [NO2]equil^2
7) Taking the square root of both sides, we get:
[NO2]equil = 0.06 mol dm^-3
So the equilibrium concentration of NO2 is 0.06 mol dm^-3.
Let me know if you have any other questions! I can also provide a more step-by-step explanation if needed.