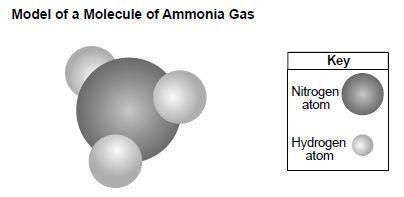
The model below represents a molecule of ammonia gas.
Ammonia gas would be classified as?
A) element
B) atom
C) compound
D) mixture
Answers
Ammonia gas is a compound that is made up of two elements, nitrogen and hydrogen. So, option C is right.
A compound is a substance that is made up of two or more elements chemically combined. Ammonia gas is made up of the elements nitrogen and hydrogen, which are chemically combined in the ratio of 1:3.
Elements are the basic building blocks of matter. They cannot be broken down into simpler substances by chemical means. Atoms are the smallest unit of an element that can exist.
Mixtures are substances that are made up of two or more elements or compounds that are not chemically combined. The components of a mixture can be separated by physical means, such as filtration or distillation.
An atom is the smallest unit of an element that can exist and ammonia gas is not an atom because it is not the smallest unit of an element.
Therefore, option C, compound is correct.
Learn more about gas here;
https://brainly.com/question/8073681
#SPJ6
Related Questions
The faster rate of reaction was caused by using a higher temperature. Explain, in terms of particles, why a higher temperature causes a faster rate of reaction.
Answers
Answer:
Increasing the temperature a reaction takes place at increases the rate of reaction. At higher temperatures, particles can collide more often and with more energy, which makes the reaction take place more quickly.
Explanation:
When 10 grams of water completely evaporate into water vapor, the volume of the water vapor is greater than the volume of the original liquid water, but the mass remains the same. Why does the mass stay the same? Which answer is correct?
A
The temperature of the liquid water and water vapor is the same.
The temperature of the liquid water and water vapor is the same.
B
The density of the liquid water is the same as the density of the water vapor.
The density of the liquid water is the same as the density of the water vapor.
C
The number of atoms in the water vapor is equal to the number of atoms in the liquid water.
The number of atoms in the water vapor is equal to the number of atoms in the liquid water.
D
The distance between the water molecules is the same in the liquid and the vapor.
The distance between the water molecules is the same in the liquid and the vapor.
Answers
Answer: I think the answer is B or C, its kind of hard.
As the number of atoms in the water vapor is equal to the number of atoms in the liquid water. Therefore, the mass of the water remains the same.
What is evaporation?Evaporation is a process that usually happens on the surface of water or liquid and it involves the conversion of the liquid phase into the gaseous phase. This process involves a change in the state of matter of water or liquids.
When the water is completely evaporated into water vapor the density of the water vapor is less than the density of the liquid water as the vapors occupy a large volume in comparison to liquid water.
But the number of atoms in the water vapor is the same as the number of atoms in the liquid water. The mass of the water is due to the mass of the atoms.
Therefore, the mass of the water remains the same during the phase transition from liquid water to water vapors. Therefore, option (C) is correct.
Learn more about evaporation, here:
https://brainly.com/question/5019199
#SPJ2
Which of these forces does not act over a distance?
A. Friction
B. Electric
C. Magnetic
D. Gravitational
Answers
Select the statements that are true about electronegativity between elements,
and their type of bond.
A. If the difference is over 1.7, an ionic bond will form.
B. If the difference in electronegativity is from 0.6 to 1.7, the bond
will be nonpolar and covalent.
C. If the difference in electronegativity is 2 to 2.5, the bond will be
nonpolar covalent.
Answers
Answer:
a
Explanation:
if the difference is over 1.7, an ionic bond will form
which of the following has more particles
6.02x10(small numbers)23 molecules CO2
9 moles PF2
10 mole NaCI
18 g H2O
Answers
Answer:
A mole is Avogadro's number of items: 6.022 × 1023.
Explanation:
2. How many carbon atoms would be in 2 MOLES of carbon?
Answers
Answer:
In one mole of carbon , there are 6.02 × 1023 atoms. so in two moles , there will be twice that = 1.204× 1024.
each side of a square is 8 by 10 metre find the area of the square
Answers
Answer and Explanation:
I believe the answer is 8
How many kilograms of water must be added to 6.07 grams of oxalic acid (H2C2O4) to make a 0.025 m solution?
Answers
M
a
V
a
=
M
b
V
b
M
a
= 6.77M - the initial molarity (concentration)
V
a
= 15.00 mL - the initial volume
M
b
= 1.50 M - the desired molarity (concentration)
V
b
= (15.00 + x mL) - the volume of the desired solution
(6.77 M) (15.00 mL) = (1.50 M)(15.00 mL + x )
101.55 M mL= 22.5 M mL + 1.50x M
101.55 M mL - 22.5 M mL = 1.50x M
79.05 M mL = 1.50 M
79.05 M mL / 1.50 M = x
52.7 mL = x
59.7 mL needs to be added to the original 15.00 mL solution in order to dilute it from 6.77 M to 1.50 M.
I hope this was helpful.
2. Name each of the following ionic compounds A. K2O B. Cacl2. C. Mg3N2 D. NaCIO E. KNO3
Answers
B. Calcium chloride
C. Magnesium nitride
D. Sodium hypochlorite
E. Potassium nitrate
Ionic compound K₂O is potassium superoxide. CaCl₂ is calcium chloride. The compound Mg₃N₂ is magnesium nitride and NaClO is sodium hypochlorite and KNO₃ is potassium nitrate.
What are ionic compounds?Ionic compounds are formed between metals and non-metals. They are bonded by lose of electrons from the metal to the non-metals. An ionic compound is named starting with the cation and then the anionic part is named second with the suffix "ide" , "ate" , "ite" etc.
The compounds with the general formula M₂O are called super oxides. K₂O is called potassium superoxide or dipotassium oxide.
The compound, CaCl₂ is named as calcium chloride. The subscripts in formula are the number of each atoms in the compounds.
Similarly Mg₃N₂ is named as magnesium nitride and KNO₃ is called potassium nitrate. The compound NaClO is named as sodium hypochlorite.
To find more on ionic compounds, refer here:
https://brainly.com/question/9167977
#SPJ5
True or false, the torch that is carried to the Olympic cauldron to be lit, is ignited by a match
Answers
Answer:
true
Explanation:
How many moles of I2 are in 8.23 moles of NaI ?
Answers
Answer: 4.12
Explanation:
we know that the given mol is 8.23 mol and they are 2NaI and I2 so we will write the equation like this.
8.23mol NaI x 1mol of I2 ÷ 2molNaI = 4.115≅ 4.12 mol of I2
we placed NaI at the bottom to cancel out with the 8.23 mol of NaI
Someone help with this question
Answers
Answer:
the answer is D the dominant over powers the resecive traits
Hey. Can someone, please help me with the following questions:
Satellites
Satellites ___________the earth.
You can see _____________ _______________ without a telescope.
An example of a man made satellite is the _____________ _____________ _____________.
The only natural satellite of the earth is the __________.
Choose from: moon, orbit, natural satellites, international space station
Planets
There are ________ planets you can see with the __________ __________.
The five planets are, _________, ___________, ___________, ___________, ___________.
The planets are part of the ___________ ___________.
Light from Saturn takes around ___________ hours to reach earth.
Choose from: 1,5, naked eye, solar system, Mercury, Venus, Mars, Jupiter, Saturn
Comets and Meteors
A _________ is a spectacular sight in the _________ sky.
They are _______ to see and could be described as giant ___________ that orbit the _______.
__________ are bits of dust and _________ that _______ up as they move through the earth’s ____________. A meteor that makes it to earth is called a ______________.
Galaxies
1. What are most of the dots of light that we see in the sky?
2. What is the name of our home galaxy?
3. What is the name of our nearest star?
4. How long does it take the light from the sun to reach the earth?
5. Can you say what some of the other dots of light in the night sky might be?
6. How many stars might there be in one galaxy?
7. What is the name of our nearest neighbor galaxy?
8. State how long it takes for the light from our neighbor galaxy to reach the earth
Answers
5, naked eye, Mercy, Venus, Mars, Jupiter, Saturn, solar system, 1
I would need to see the choices for this section
1.Stars
2. The Milky Way
3. The sun
4.8 minutes
5. Satellites
6. 100 thousand million
7. Canis Major Dwarf Galaxy
8. 10s of thousands of years
What is the molar mass of the following compound: C1H3P2
Answers
Please help me find the final answer
Answers
Answer:
5. The mass of Na₂CO₃, that will produce 5 g of CO₂ is approximately 12.04 grams of Na₂CO₃
6. The mass of nitrogen gas (N₂) that will react completely with 150 g of hydrogen (H₂) in the production of NH₃ is 693.[tex]\overline{3168}[/tex] grams of N₂
Explanation:
5. The given equation for the formation of carbon dioxide (CO₂) from sodium bicarbonate (Na₂CO₃) is presented as follows;
(Na₂CO₃) + 2HCl → 2NaCl + CO₂ + H₂O
One mole (105.99 g) of Na₂CO₃ produces 1 mole (44.01 g) of CO₂
The mass, 'x' g of Na₂CO₃, that will produce 5 g of CO₂ is given by the law of definite proportions as follows;
[tex]\dfrac{x \ g}{105.99 \ g} = \dfrac{5 \ g}{44.01 \ g}[/tex]
[tex]\therefore {x \ g} = \dfrac{5 \ g}{44.01 \ g} \times 105.99 \ g \approx 12.04 \ g[/tex]
The mass of Na₂CO₃, that will produce 5 g of CO₂, x ≈ 12.04 g
6. The chemical equation for the reaction is presented as follows;
N₂ + 3H₂ → 2NH₃
Therefore, one mole (28.01 g) of nitrogen gas, (N₂), reacts with three moles (3 × 2.02 g) of hydrogen gas (H₂) to produce 2 moles of ammonia (NH₃)
The mass 'x' grams of nitrogen gas (N₂) that will react completely with150 g of hydrogen (H₂) in the production of NH₃ is given as follows;
[tex]\dfrac{x \ g}{28 .01 \ g} = \dfrac{150 \ g}{3 \times 2.02 \ g} = \dfrac{150 \ g}{6.06 \ g}[/tex]
[tex]\therefore \ x \ g= \dfrac{150 \ g}{6.06 \ g} \times 28.01 \ g = 693.\overline {3168} \ g[/tex]
The mass of nitrogen gas (N₂) that will react completely with 150 g of hydrogen (H₂) in the production of NH₃, x = 693.[tex]\overline{3168}[/tex] grams
HELPPP ASAPPP
Use the element tile below to calculate the molar mass of He2 (helium gas).
2 g/mol
16.012 g/mol
8.006 g/mol
4.003 g/mol
Answers
Answer: 4.003 g/mol
Explanation:
So basically Molar mass is the atomic mass of an element and the atomic mass of Helium is 4.0026 or 4.003 g/mol
multiply the molar mass of helium (4.003) by the subscript and you have your answer!
3. What tool do we use to measure force?
Answers
Answer:
A force meter
what is a salient factor?
Answers
Answer:
technology is a silent factor
Explanation:
learned about it in computer class.
K(s)+H2SO4(aq)
——>_____+______
Answers
K2SO4 + H2
Reason :Potassium is highly reactive
Answer:
K2SO4 + H2 this is the reaction.
Calculate the number of moles of gas that occupy a 16L container at a pressure of 3 atm and a temperature of 48oC
Answers
Answer:
1.81
Explanation:
3*16=n(0.0821)(48+273)
What is the limiting reactant if 10 moles of NH 3 react with 30.0 moles of NO ?
Answers
Answer:
NO is the limiting reactant.
Explanation:
4NH3 + 6NO --> 5N2 + 6H2O
Molar ration NH3 : NO = 10: 30 = 1: 3
Stoichiometric molar ration NH3:NO = 4:6 = 2:3
NO is the limiting reactant.
For the reaction between aqueous silver nitrate and aqueous sodium chloride, write each of the following. The products of the reaction are aqueous sodium nitrate and solid silver chloride.
Complete equation
Complete ionic equation
Net ionic equation
Answers
Answer:
A balanced ionic equation shows the reacting ions in a chemical reaction. These equations can be used to represent what happens in precipitation reactions or displacement reactions.
Precipitation reactions
In a typical precipitation reaction, two soluble reactants form an insoluble product and a soluble product.
For example, silver nitrate solution reacts with sodium chloride solution. Insoluble solid silver chloride and sodium nitrate solution form:
AgNO3(aq) + NaCl(aq) → AgCl(s) + NaNO3(aq)
The Na+ ions and NO3- ions remain separate in the sodium nitrate solution and do not form a precipitate. Ions that remain essentially unchanged during a reaction are called spectator ions.This means these can be ignored when writing the ionic equation. Only how the solid silver chloride forms is needed to be shown:
Ag+(aq) + Cl-(aq) → AgCl(s)
In a balanced ionic equation:
the number of positive and negative charges is the same
the numbers of atoms of each element on the left and right are the same
Displacement reactions
Displacement reactions take place when a reactive element displaces a less reactive element from one of its compounds.
A common type of displacement reaction takes place when a reactive metal reacts with the salt of a less reactive metal. For example, copper reacts with silver nitrate solution to produce silver and copper(II) nitrate solution:
2AgNO3(aq) + Cu(s) → 2Ag(s) + Cu(NO3)2(aq)
In this reaction, the NO3- ions remain in the solution and do not react - they are the spectator ions in this reaction. So, they can be removed from the ionic equation:
2Ag+(aq) + Cu(s) → 2Ag(s) + Cu2+(aq)
Question
Explain why this ionic equation is balanced:
Ba2+(aq) + SO42-(aq) → BaSO4(s)
Hide answer
There are the same numbers of atoms of each element on both sides of the equation. The total charge on both sides is also the same (zero).
Question
Balance this ionic equation, which represents the formation of a silver carbonate precipitate:
Ag+(aq) + CO32-(aq) → Ag2CO3(s)
Hide answer
2Ag+(aq) + CO32-(aq) → Ag2CO3(s)
Question
Balance this ionic equation, which represents the displacement of iodine from iodide ions by chlorine:
Cl2(aq) + I-(aq) → I2(aq) + Cl-(aq)
Hide answer
Cl2(aq) + 2I-(aq) → I2(aq) + 2Cl-(aq
Explanation:
this will help, I used this for my work x
AgNO₃ (aq) + NaCl (aq) → AgCl (s) + NaNO₃(aq) is the complete chemical equation taking place between the 2 reactants.
Chemical equation is a symbolic representation of a chemical reaction which is written in the form of symbols and chemical formulas.The reactants are present on the left hand side while the products are present on the right hand side.
A plus sign is present between reactants and products if they are more than one in any case and an arrow is present pointing towards the product side which indicates the direction of the reaction .There are coefficients present next to the chemical symbols and formulas .
Learn more about chemical equation,here:
https://brainly.com/question/28792948
#SPJ6
The chemical equation below represents a reversible reaction.
H2PO4- + H2O ↔ H3PO4 + OH-
Which pair represents an acid and its conjugate base?
Answers
Answer:b
Explanation:
bc
In the given chemical equation, H₃PO₄ is an acid while it's conjugate base is OH⁻.
What is chemical equation?Chemical equation is a symbolic representation of a chemical reaction which is written in the form of symbols and chemical formulas.The reactants are present on the left hand side while the products are present on the right hand side.
A plus sign is present between reactants and products if they are more than one in any case and an arrow is present pointing towards the product side which indicates the direction of the reaction .There are coefficients present next to the chemical symbols and formulas .
The first chemical equation was put forth by Jean Beguin in 1615.By making use of chemical equations the direction of reaction ,state of reactants and products can be stated. In the chemical equations even the temperature to be maintained and catalyst can be mentioned.
Learn more about chemical equation,here:
https://brainly.com/question/28294176
#SPJ3
Nitric monoxide (NO) reacts with oxygen gas to form nitrogen dioxide (NO₂), a dark brown gas. If 5.895 mol of NO is mixed with 2.503 mol of O₂,
determine the limiting reagent.
calculate the number of grams of NO₂ produced.
and determine how many grams of excess reagent remain unreacted.
Answers
Answer:
Limiting reactant: O2
grams NO2 produced = 230.276 g NO2
grams of NO unused = 26.67 gNO
Explanation:
2NO + O2 --> 2NO2
Step 1: Determine the molar ratio NO:O2
molar ratio NO:O2 = 5.895: 2.503 = 2.35
stoichiometric molar ratio NO:O2 = 2:1
So, O2 is the limiting reactant.
Step2: Determine the grams of NO2:
?g NO2 = moles O2 x (2moles NO2/1 mol O2) x (MM NO2/ 1 mol NO2) = 2.503 x 2 x 46 = 230.276 g NO2
Step 3: Determine the amount of excess reagent unreacted
moles excess NO reacted = moles O2 x (2 moles NO/1 mol O2) = 2.503 x 2 = 5.006 moles NO reacted
moles NO unreacted = total moles NO - moles NO reacted = 5.895-5.006 =0.889 moles NO unreacted
mass NO unreacted = moles NO unreacted x MM NO = 0.889 x 30 =26.67 g NO unreacted
how would I solve this problem?
Answers
Answer:
D
Explanation:
The goal in this set up is to make sure all units cancel each other.
Since we need to go from grams to atoms, our final unit should be atoms.
Using method of elimination, let's see why other options are wrong.
A - Although 'g' is cancelled out, we are left with moles^2 / atoms
B - Although 'g' is cancelled out, we are left with moles^2 / atoms
C - Although 'mol' is cancelled out, we are left with g^2 / atoms
Calculate the percent composition of oxygen in silicon dioxide.
Answers
Answer:
then it is 5
Explanation:
because i said it is
Why do metal boats float?
Answers
Answer:
I hope this will help you
Explanation:
The air that is inside a ship is much less dense than water. That's what keeps it floating! The average density of the total volume of the ship and everything inside of it (including the air) must be less than the same volume of water.
Please make me brilliant
Carbon is one of a few elements that bonds to itself. The scientific term for this is
Answers
Answer:
Alkanes
Explanation:
Explanation:
The answer is Alkanes.Alkanes are compound that consists entirely of atoms of carbon and hydrogen joined to one another by single bonds
Which element would you predict to have the highest first ionization energy? Why?
a) neon or carbon
b) fluorine or francium
c) beryllium or strontium
d) iodine or fluorine
Answers
Describe a time in the group discussion when you referred to the notes you took, the graphic organizer you filled out, and your position paper.
Answers
Answer:
To back up any statements I make.
To refute any errors presented.
Explanation:
During a group discussion, you look at your notes to point out an error or when you want to flesh out a point you made.
What is a group discussion?The term group discussion has to do with learners talking about a topic in a manner ion which each learner takes a turn to explain a portion of the topic.
During a group discussion, you look at your notes to point out an error or when you want to flesh out a point you made.
Learn more about group discussion:https://brainly.com/question/11940982
#SPJ2