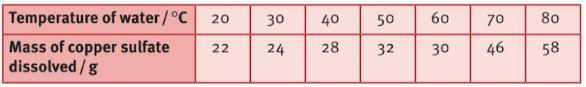
Sam and Jane have been investigating the amount of copper sulphate that can be dissolved in water at different temperatures .They added copper sulphate till it could not be dissolved any further and also measured the mass of copper. The results are below. What conclusions can you make from results?
Answers
Answer:
This question is so confusing, I'm sorry
Related Questions
Limiting Reactant
12.0 grams of sodium reacts with 5.00 grams of chlorine. What mass of sodium
chloride could be produced?
Nas) +
Cl2(g) →
NaCls)
(1)
(2)
Identify the limiting reactant.
Determine the amount of sodium chloride produced.
Answers
Answer:
(1) Cl₂ is the limiting reactant.
(2) 8.18 g
Explanation:
2Na(s) + Cl₂(g) → 2NaCl(s)First we convert the given masses of reactants into moles, using their respective molar masses:
Na ⇒ 12.0 g ÷ 23 g/mol = 0.522 mol NaCl₂ ⇒ 5.00 g ÷ 70.9 g/mol = 0.070 mol Cl₂0.070 moles of Cl₂ would react completely with (2 * 0.070) 0.14 moles of Na. There are more Na moles than that, so Na is the reactant in excess while Cl₂ is the limiting reactant.
Then we calculate how many moles of NaCl are formed, using the limiting reactant:
0.070 mol Cl₂ * [tex]\frac{2molNaCl}{1molCl_2}[/tex] = 0.14 mol NaClFinally we convert NaCl moles into grams:
0.14 mol NaCl * 58.44 g/mol = 8.18 gHydrogen can be produced according to the following word equation
=zinc +hydrochloric acid =zinc chloride +hydrogen [zn^+2]
Write a complete balanced chemical equation for this chemical reaction?
Answers
Answer:
Zn + 2HCl → ZnCl₂ + H₂
Explanation:
First we write the equation using the molecular formulas instead of words:
Zn + HCl → ZnCl₂ + H₂We know zinc chloride is ZnCl₂ as the problem tells us the oxidation state of zinc in the products is +2, and chloride means Cl⁻¹.
Now we proceed to balance the reaction:
There are 2 Cl atoms and 2 H atoms on the right side, so we add a coefficient of 2 to HCl on the left side:
Zn + 2HCl → ZnCl₂ + H₂find the sum of 15, 9, 3, ....... 45
Answers
Answer:
hope this helps
Explanation:
72
4. What is the specific heat of a substance if 5800 joules is
released in a 250 gram sample that will cool the substance from
60 degrees to 45 degrees?
Answers
Answer: 23.2
Explanation:
3. Explain what would happen to the digestion process if enzymes were not present. SC.6.L.14.5
Answers
What are the details of the chemical structure of methylisothiazolinone (MIT)?
Answers
Which of the following statements best describes a
mixture?
O A substance that contains two or more elements chemically joined
O A substance that contains two or more elements not chemically joined
O A substance that contains only one type of atom
O A substance that contains two or more compounds or elements that are
not chemically joined.
Answers
In the UNBALANCED chemical reaction for the combustion of acetylene (used in welding torches), determine at standard temperature and pressure, how many liters of
H2O gas are produced if 12 liters of Oxygen gas are completely consumed?
__C2H2 + __O2 —> __CO2 + __H2O
(Please help! Random answers for points will be reported)
Answers
Answer:
2 C2H2 + 5 O2 = 4 CO2 + 2 H2O
I've checked this multiple times this should be it
Name the functional group in the
following molecule:
HSCH2CH3
A. alkane
B. amide
C. thiol
D. alkyl halide
Answers
Answer:
C. thiol
Explanation:
Answer:
it's C thiol I just took the test
hope this helps
have a good day :)
Explanation:
Which change represents an oxidation reaction?
Answers
What is the concentration of a 22.35 L solution that contains 9.95 moles of sodium
acetate? Round your answer to the correct number of significant figures.
Answers
Answer:
0.445 M
Explanation:
Molarity = moles/Volume
M = 9.95/22.35 = 0.445 M
The type of potential energy related to an object's height
Answers
Answer:
the answer is gravitational potential energy
Answer:
Since the gravitational potential energy of an object is directly proportional to its height above the zero position, a doubling of the height will result in a doubling of the gravitational potential energy. A tripling of the height will result in a tripling of the gravitational potential energy.
Explanation:
Hope this helps?
Which is a form of kinetic energy?
A. gravitational energy
B. chemical energy
C. electrical energy
D. sound energy
Answers
Which phrase describes air density?
increases as altitude increases
equals mass divided by volume
pushes molecules in one direction
exerts less pressure as it increases
If you answer I love you
Answers
Answer:B equals mass divided by volume
Explanation:I got hacks :)
Answer:
Which phrase describes air density? well b
increases as altitude increases
equals mass divided by volume
pushes molecules in one direction
exerts less pressure as it increases
Explanation:
love you too!!!!!
what is the structure of methane
Answers
Answer:
CH4
Explanation:
if it is wrong, i blame my brain
Fill in the blanks. 3NH3
Answers
Answer:
3, 9, 3
Explanation:
The coefficient of 3 tells us that there are three molecules (the chemical unit of NH3). Each molecule of ammonia (NH3) is made up of 1 atom of nitrogen bonded to 3 atoms of hydrogen.
Since there are three molecules, we have three times the amount of atoms there are in one molecule.
3 x 1 = 3 nitrogen
3 x 3 = 9 hydrogen
If I began this reaction with 1.7g of O2 , how much water would I make
Answers
Lewis dot diagram for the Cs1+ ion
Answers
Answer:
[tex]Cs^+[/tex]
Explanation:
Cesium Lewis dot structure would look like this:
·Cs, because it only has one valence electron. But, since it has a plus, that means we lost an electron. So, we have to get rid of the dot and you have:
[tex]Cs^+[/tex]
How are humidity and precipitation related?
A. When humidity is low, the chance of precipitation is high.
B. When humidity is high, the chance of precipitation is high.
C. When humidity is high, the chance of precipitation is low.
D. Changes in humidity do not cause precipitation.
Answers
If you have 9.56 moles of aluminum oxide how many moles of sodium oxide could you produce?
Answers
Answer:
The answer is 28.68 moles
Explanation:
Hope this helped Mark BRAINLEST!!!
Rock is driven underground and changed by heat and pressure. This describes
what?
a. Igneous changing to sedimentary
b. Metamorphic changing to sedimentary
C. Sedimentary changing to metamorphic
d. Sedimentary changing to igneous
Answers
Answer:
Explanation:
metamorphic
Help plz now !
Which statement explains why a chemical equation must be balanced?
A. It must show the reactants and products on the correct sides of
the reaction arrow.
B. It must show that the mass of each element involved is conserved
by a chemical reaction.
C. It must show how each chemical formula is written to accurately
represent each substance.
D. It must show that coefficients and subscripts can be used in
chemical formulas.
Answers
Answer: B. It must show that the mass of each element involved is conserved by a chemical reaction.
Explanation:
According to the law of conservation of mass, mass can neither be created nor be destroyed. Thus the mass of products has to be equal to the mass of reactants. The number of atoms of each element has to be same on reactant and product side. Thus chemical equations are balanced.
The correct statement why a chemical equation must be balanced is It must show that the mass of each element involved is conserved by a chemical reaction.
Answer:
B. It must show that the mass of each element involved is conserved
by a chemical reaction.
Explanation:
a p e x, just took the quiz
Draw the structure formula for
1-ethyl-2,3-dimethylbenzene
Please help me
Answers
Answer:
Please see the attached picture.
Explanation:
1) Draw a benzene ring
2) On the first carbon, add a CH₂CH₃ (known as ethyl) since its location number is 1.
3) 'Dimethyl' refers to 2 methyl (CH₃) branches. The location numbers are 2 and 3, thus add a CH₃ on the 2nd and 3rd carbons.
Use the solubility rules from the Lab 4 introduction and your knowledge of qualitative separation schemes from the lab to answer the following questions. The qualitative analysis experiment you did is actually an abbreviated version of a much larger analysis scheme in which many different cations are separated and identified. Suppose a mixture contains Ag , K , NH4 , Hg22 , Pb2 , Mg2 , Sr2 , Ba2 , Cu2 , Al3 and Fe3 .
(a) Which of the following ions could you separate, by causing them to precipitate, with the addition of HCl?
Ag+ K+ NH4+
Hg22+ Pb2+ Mg2+
Sr2+ Ba2+ Cu2+
Al3+ Fe3+
(b) After the addition of HCl, the above sample is centrifuged and decanted. Which of the following cations remaining in the supernatant could you separate, by causing them to precipitate, with the addition of H2SO4? (Hint: H2SO4 is a source of sulfate ions. Select all that apply.)
Ag+ K+ NH4+
Hg22+ Pb2+ Mg2+
Sr2+ Ba2+ Cu2+
Al3+ Fe3+
Answers
Answer:
a13+a13
Explanation:
2. I need to find the angle of corner of a room, what tool could I use?
a. Combination square
b. Plumb-bob
c. Sliding t-bevel
d. Tri-square
Answers
Which statement BEST describes why licensed health care workers are held to a higher standard?
A. They agree to ethical standards when they are licensed.
B. They agree to be good role models and always model good habits.
C. They are more involved than the public.
D. They are more educated than most of the public.
Answers
Answer:
They agree to ethical standards when they are licensed.
Explanation:
Answer:
C) They agree to ethical standards when they are licensed
Explanation:
g Hydrogen iodide, HI, decomposes at moderate temperatures according to the equation The amount of I2 in the reaction mixture can be determined from the intensity of the violet color of I2; the more intense the color, the more I2 in the reaction vessel. When 3.80 mol HI was placed in a 5.00-L vessel at a certain temperature, the equilibrium mixture was found to contain 0.443 mol I2. What is the value of Kc for the decomposition of HI at this temperature
Answers
Answer:
Equilibrium constant, Kc = 0.023
Explanation:
Equation for the decomposition of Hydrogen iodide is given below:
2HI ----> H₂ + I₂
Initially, the number of moles of the reactant and the products are given as follows:
n(HI) = 2 * 3.800 moles = 7.600 moles
nH₂) = 0.000 moles
n(I₂) = 0.000 moles
At equilibrium, the equation becomes: 2HI <----> H₂ + I₂
Number of moles of the reactant and the products are given as follows:
n(HI) = 7.600 - (0.886 + 0.886) moles = 5.828 moles
nH₂) = 2 * 0.443 = 0.886 moles
n(I₂) = 2 * 0.443 = 0.886 moles
Equilibrium constant, Kc = [H₂] [I₂] / [HI]²
Equilibrium constant, Kc = (0.886) * (0.886) / (5.828)²
Equilibrium constant, Kc = 0.023
What does this even mean? Help please and thank you!
Answers
Answer:
T₁ = P₁V₁T₂ / P₂V₂
Explanation:
The combine gas equation is given by:
P₁V₁ / T₁ = P₂V₂ / T₂
Where:
P₁ => is the initial pressure
V₁ => is the initial volume
T₁ => is the initial temperature
P₂ => is the final pressure
V₂ => is the final volume
T₂ => is the final temperature
Finally, we shall make T₁ the subject of the above expression. This can be obtained as follow:
P₁V₁ / T₁ = P₂V₂ / T₂
Cross multiply
T₁P₂V₂ = P₁V₁T₂
Divide both side by P₂V₂
T₁ = P₁V₁T₂ / P₂V₂
Thus, the formula for T₁ is:
T₁ = P₁V₁T₂ / P₂V₂
How many molecules are in 450.0 grams of aluminum fluoride(AIF3)?
Answers
Answer:
It would be exactly 5.3586262014272155. But if you were to round it up it would be 5.35.
I need help with like 35 questions anyone willing to help please let me know I have discord
UnknownGoddxss#2795
Please I only have like 5 hours to complete this
Answers
Answer and Explanation:
The molar mass of a substance is all of the weights from the elements combined.
So, we have the elements
CU, S, and [tex]O_{4}[/tex]
CU has a mass is 65
S mass is 32
O has a mass of 16, but there's 4 atoms of O, so we do 16 times 4, which is 64.
Now we add.
65 + 32 + 64 is 161.
So, the answer is 160, or answer choice A.
#teamtrees #PAW (Plant And Water)