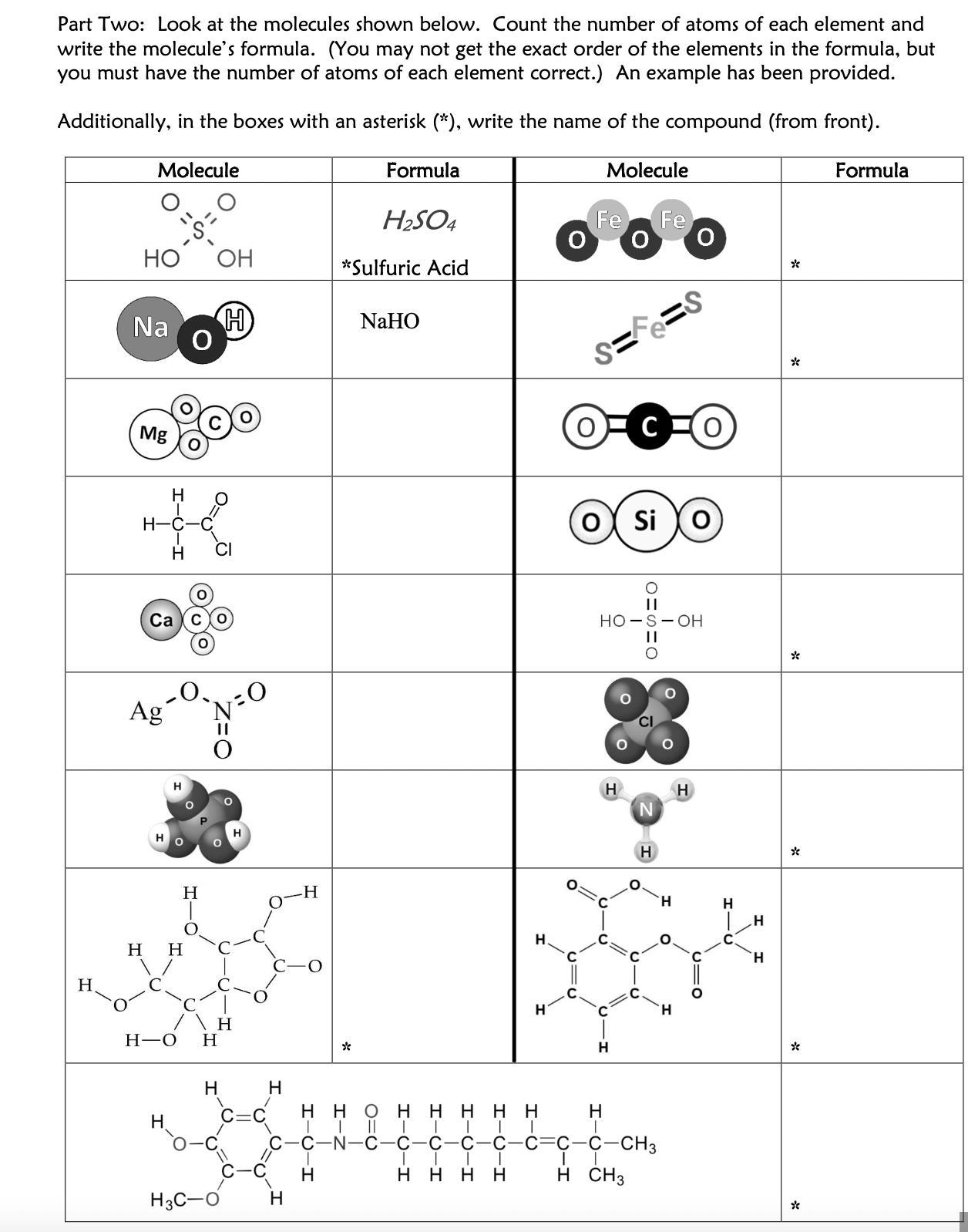
PLS HURRY TY top says "Part Two: Look at the molecules shown below. Count the number of atoms of each element and write the molecule’s formula. (You may not get the exact order of the elements in the formula, but you must have the number of atoms of each element correct.) An example has been provided. Additionally, in the boxes with an asterisk (*), write the name of the compound (from front). "
Answers
Answer: capsaicin C18 H27 NO3
Explanation: I just know the last one sorry G
Related Questions
4. Based on their placement on the periodic table, which of the following is more
reactive?
A.Sodium (Na)
B. Magnesium (Mg)
C. Barium (Ba)
D. Neon (Ne)
Answers
Propane is an alkene. Describe the test for alkenes. Give the colour change in the test.
Answers
Answer:
Explanation:
One test for Alkenes is testing with bromine water. This test can be used to tell the difference between an Alkanes and Alkenes. On a normal, any Alkene will turn brown bromine water to colourless because the bromine reacts with the double carbon-carbon bond. Also, the reaction takes place with unsaturated compounds that contains the double carbon-carbon bonds.
Conversely, when Alkanes go through the same test, there is no reaction with bromine water.
A sample of krypton occupies 15.0 L at a pressure of 2.1 atm. Use Boyle's Law to find the pressure of the krypton when the volume is decreased to 4L.
Answers
Answer:
7.5 atm
Explanation:
Given that,
Initial volume, V₁ = 15 L
Pressure, P₁ = 2.1 atm
Final volume, V₂ = 4L
We need to find the final pressure. The mathematical relation between volume and pressure is given by :
[tex]\dfrac{V_1}{V_2}=\dfrac{P_2}{P_1}\\\\P_2=\dfrac{V_1P_1}{V_2}\\\\P_2=\dfrac{15\times 2}{4}\\\\P_2=7.5\ atm[/tex]
So, the final pressure is equal to 7.5 atm.
TVINN
Assertion: Naphthalene,
camphor, iodine, ammonium
chloride are some common
examples of the substances
which undergo sublimation.
Reason: All solids are first
converted to liquids and then
gases on heating.
Both assertion (A) and
Reason (R) are true
and Reason (R) is the
correct explanation of
assertion (A)
Both assertion (A) and
reason (R) are true but
reason (R) is not the
correct explanation of
assertion (A)
w
Assertion (A) is true
but reason (R) is false.
Assertion (A) is false but reason (R)is true.
Answers
Answer:
Assertion (A) is true
but reason (R) is false.
Explanation:
If we look at the assertion closely, it is true that a sublime substance changes from solid to gas without passing through a liquid state. Naphthalene, camphor, iodine and ammonium chloride are some common examples of the substances which undergo sublimation.
However, not all solids first pass through a liquid state before converting to gas. Sublime solids such as those listed above passes directly from solid to gas without passing through a liquid state.
Hence the assertion is true but reason is false.
If 2.28 g C8H18 reacts with 7.00 g of Oz, predict the mass of CO, that can be produced and
identify the limiting reactant. Explain how you got your answers.
Unbalanced equation: C8H180 + 026) • CO26) + H₂O
Answers
Answer:
O₂ is limiting reactant and 6.17g CO₂ can be produced
Explanation:
Based on the balanced reaction:
C₈H₁₈ + 25/2O₂ → 8CO₂ + 9H₂O
1 mole of C₈H₁₈ reacts with 25/2 moles O₂.
To solve this question we must convert the mass of each reactant to moles and, using the balanced reaction, we can find limiting reactant. With moles of limiting reactant we can find the moles of CO₂ and its mass as follows:
Moles C₈H₁₈ -Molar mass: 114.23g/mol-
2.28g * (1mol / 114.23g) = 0.0200 moles C₈H₁₈
Moles O₂ -Molar mass: 32g/mol-
7.00g * (1mol / 32g) = 0.219 moles O₂
For a complete reaction of 0.0200 moles C₈H₁₈ are needed:
0.0200 moles C₈H₁₈ * (25/2moles O₂ / 1mole C₈H₁₈) = 0.250 moles of O₂ are needed.
As there are just 0.219 moles, O₂ is limiting reactant.
Moles of CO₂ that can be produced:
0.219 moles O₂ * (8moles CO₂ / 25/2moles O₂) = 0.140 moles CO₂
The mass is -Molar mass CO₂: 44.01g/mol-:
0.140 moles CO₂ * (44.01g / mol) =
6.17g CO₂ can be producedHow many grams are in 0.428 moles of lithium carbonate, Li3PO4?
42.7
49.6
54.8
72.3
Answers
Which expression represents the pH of a solution?
Answers
Answer:
pH = 14 - pOH
pH = -log[H+]
pH = pKa + log [A-]/[HA]
Explanation:
The equation used depends on what is given in the equation.
The expression that represent the pH of the solution is
pH = 14 - pOH
pH = -log[H+]
pH = pKa + log [A-]/[HA]
What is the pH of the solution?It is the quantitative measure related to the acidity or the other liquid solutions. It should be range between the 1 and 10^-14. here the numbers be like from 0 to 14.
Hence, we can say that The expression that represent the pH of the solution is
pH = 14 - pOH
pH = -log[H+]
pH = pKa + log [A-]/[HA]
Learn more about pH here: https://brainly.com/question/12052751
teaching self-regulation begins with helping a child
Answers
Answer:
hey jazmin! I have been on your friends list for a while on brainly, I thought I'd say hi.
How many moles is 8.3 x 10^12 atoms or particles of Br2
urgent
Answers
Explanation:
To find the number of moles in a substance given it's number of entities we use the formula
[tex]n = \frac{N}{L} \\[/tex]
where n is the number of moles
N is the number of entities
L is the Avogadro's constant which is
6.02 × 10²³ entities
We have
[tex]n = \frac{8.3 \times {10}^{12} }{6.02 \times {10}^{23} } \\ \\ \\ \\ \: \: \: \: \: \: \: = 1.379 \times {10}^{ - 11} \: \: \: \: moles[/tex]
Hope this helps you
A reaction yield 6.26 g of a product what is the percent yield if the theoretical yield is 18.81 g
Answers
Explanation:
remember the equation percentage yield = actual/theoretical yield
so 6.26/18.81 X 100 gives u 33.28017012 so to 3 SF ot would be 33.3%
hope this helps:)
The statement, that describes the percent yield is 33.280 g.
What is percent yield?The percent yield is the ratio of actual yield to theoretical yield. It is computed by dividing the experimental yield by the theoretical yield and multiplying the result by 100 percent. If the actual and theoretical yields are equal, the percent yield is one hundred percent.
To express the efficiency of a reaction, use the following formula: percent yield
= (actual yield/theoretical yield) x 100
= (6.26 g/ 18.81 g) x 100
= 33.280 g
Hence the correct answer is 33.280 g.
Learn more about percent yield here
https://brainly.in/question/5487028
#SPJ2
What is the balanced equation for combustion of copper (I)?
Answers
Answer:
Cu + O2 = CuO - Chemical Equation Balancer.The number of molecules in 1.00 liter of O2 gas at 56◦C and 821 torr is
1. 1.83 × 1025 molec.
2. 32 molec.
3. 2.24 × 1023 molec.
4. 2.41 × 1022 molec.
5. 4 × 10−2 molec.
Answers
Answer:
2. 2.41 x 10^22 molec
Explanation:
Answer:
2. 2.41 x 10^22 molec
Explanation:
the other person said that and i got right on my quiz
What is the density of an object that has a mass of 350 g and a volume of 95 cm?? Would
this object float in water?
Answers
Answer:
Density= 3.68g/cm³
The object will sink in water
Explanation:
D=m/v
=350/95
=3.684
≈3.68g/cm³
Water= 1g/cm³
The object will sink in water because the object is denser than the water.
3.684g/cm³ is the density of an object that has a mass of 350 g and a volume of 95 cm³. The object will sink in water.
What is density?The density of a material (volumetric density and specific mass) is its mass per unit volume. The most common sign for density is however the Roman letter D can be used as well. Density is defined mathematically as mass divided with volume.
The density of a pure material has the same numeric values just like its mass concentration. Various materials have varied densities, and density can be important in terms of buoyancy, purity, and packing.
D=m/v
=350/95
=3.684g/cm³
The object will sink in water
Therefore, 3.684g/cm³ is the density of an object that has a mass of 350 g and a volume of 95 cm³. The object will sink in water.
To learn more about density, here:
https://brainly.com/question/29775886
#SPJ2
Why is it important for the kitchen manager to make sure food is fresh?
Answers
Which of the following do not contribute to weathering?
A. Rain
2.earthquakes
3. ice
4. wind
Answers
Answer:
Earthquakes
Explanation:
i n the notatation,5N2 what is the number of nitogen atoms
Answers
Answer:
10 nitrogens
Explanation:
The 2 is the number of nitrogens in a molecule of N2. There are 2 of them.
The 5 is a whole different critter. It tells you that it was part of an equation and it took 5 molecules of Nitrogen to balance the equation. You only see numbers to the right of a molecule when the molecule is in an equation.
5 (2)=10
what are the three method used to treat water ?
Answers
Answer:
The three methods used to treat water are
Filtration SedimentationDistillationExplanation:
These methods used include physical processes, biological processes such as slow sand filters or biologically active carbon; chemical processes such as flocculation and chlorination; and the use of electromagnetic radiation such as ultraviolet light.
15.True or False:
The concentration of a solution can be increased indefinitely by
adding solute
Answers
(I hate the 20 character limit)
How much energy does a copper sample absorb as heat if its specific heat is 0.384 J/g.°C, its mass is 8.00 g, and it is
heated from 10.0°C to 40.0°C?
Answers
Answer:
.0016
Explanation:
For specific heat problems you use the formula :
Q = mcΔT
They've given you the m, c and delta T, so you can plug in those values. (for the temperature change subtract 10 from 40 to see that it grew 30 degrees)
You're looking for Q so leave that variable in the equation. Then solve.
.Q = (8.00)(.384)(30)
Q = 92.16
92.16 J energy will be absorbed by a copper sample.
Given:
Specific heat capacity, C= 0.384 J/g °C
Temperature, T₁ = 10.0°C and T₂= 40.0°C
Mass, m=8.00 g
We know that,
The specific heat capacity is defined as the quantity of heat (J) absorbed per unit mass (g) of the material when its temperature increases or 1 °C, and its unit is J/g °C.
Heat energy in terms of specific heat energy can be calculated by using this formula:
Q= m. C. ΔT
∵ΔT= change in temperature,
ΔT=40.0-10.0°C=30°C
Now substituting the values in the above formula.
[tex]Q= 8.0*0.384*30=92.16J[/tex]
Hence, 92.16 J energy will be absorbed by a copper sample.
Learn more:
brainly.com/question/22991121
an organism that hunts other organisms for food is a
A. prey
B. predator
C. parasite
D. host
Answers
Answer:
B) a predator
What is the pressure of 13.7 mol of acetylene in a 48.1 L cylinder at 67.0 °C?
Answers
Answer:
hinojosa has caught you cheating
Explanation:
ur mom
\
Which type of reaction does an element replace an ion in a compound, forming a new compound and new element?
a
Synthesis
b
Decomposition
c
Single Replacement
d
Double Replacement
e
Combustion
Answers
Answer:
The answer is c . Single Replacement
Explanation:
A single replacement reaction, sometimes called a single displacement reaction, is a reaction in which one element is substituted for another element in a compound.
What structure behind the eardrum touch that membrane?
Answers
Answer:
The middle ear is the portion of the ear internal to the eardrum, and external to the oval window of the inner ear. The mammalian middle ear contains three ossicles, which transfer the vibrations of the eardrum into waves in the fluid and membranes of the inner ear.
Explanation:
A rocket launches because of __________.
A. an opposite reaction
B. it's large mass
C. its gravity
D. air resistance
Answers
Answer:
an opposite reaction
Earth's gravity is still pulling down on the rocket. When a rocket burns propellants and pushes out exhaust, that creates an upward force called thrust. To launch, the rocket needs enough propellants so that the thrust pushing the rocket up is greater than the force of gravity pulling the rocket down.
Which reactant is necessary for a combustion reaction?
A. Water
O B. Heat
O c. Carbon dioxide
D. Oxygen
Answers
The reactant necessary for a combustion reaction is oxygen.
(option D).
Combustion is a chemical reaction that occurs when a substance reacts with oxygen, producing heat and light. In order for combustion to take place, three elements are needed: fuel, heat, and oxygen.
Fuel is the substance that undergoes combustion, such as gasoline, wood, or methane. Heat is the energy required to initiate and sustain the reaction. Lastly, oxygen is the reactant that combines with the fuel, allowing it to burn and release energy.
When a combustion reaction occurs, the fuel and oxygen combine to form carbon dioxide (option C) and water (option A). This process is exothermic, meaning it releases energy in the form of heat and light.
For example, when wood is burned in the presence of oxygen, it undergoes combustion. The heat from a match or a spark provides the necessary activation energy to start the reaction. Oxygen from the air combines with the carbon in the wood, producing carbon dioxide and releasing energy in the form of heat and light.
In summary, oxygen is the reactant necessary for a combustion reaction. It combines with the fuel and releases energy in the form of heat and light.
(option D).
For more such questions on combustion reaction
https://brainly.com/question/13251946
#SPJ8
Explain what will happen to the organisms that is affected by human activities.
Answers
Answer:
Human activity is by far the biggest cause of habitat loss.
Humans are now responsible for causing changes in the environment that hurt animals and plant species. We take up more space on Earth for our homes and cities. We pollute habitats. ... Human activity often changes or destroys the habitats that plants and animals need to survive.
Explanation:
Human activities are the various actions for recreation, living, or necessity done by people. For instance it includes leisure, entertainment, industry, recreation, war, and exercise.
Over time, destruction of such habitats leads to reduced biodiversity, weakening the Earth's ecosystems, and ultimately posing a major threat to human life. While, significant tracts of habitat have been lost, and along with them many species of plant and animal, steps can be taken to slow and even reverse the process.
One of the most significant ways in which humans have impacted on the lives of other species is by causing climate change. Many animals, including birds and frogs, now breed much earlier in spring in Britain than they did 20 years ago. Whether or not this is having a harmful effect on them has yet to be determined.
En una botella hay 2x10 25 moléculas de vinagre puro ¿Cuántos mol y cuántos gramos de esta sustancia habrá en la botella?
Answers
Answer:
33.2 moles y 1994 gramos.
Explanation:
¡Hola!
En este caso, dado que es posible para nosotros relacionar moles con gramos por medio de la masa molar y moléculas con moles por medio del número de Avogadro, resulta factible para nosotros primero calcular las moles en las moléculas dadas y de esta manera luego calcular los gramos, teniendo en cuenta una masa molar de 60.05 g/mol ya que el vinagre se puede estudiar como ácido acético (CH3COOH):
[tex]2x10^{23}molec*\frac{1mol}{6.022x10^{23}molec}=33.2mol[/tex]
Seguidamente, calculamos los gramos:
[tex]33.2mol*\frac{60.05g}{1mol}=1994g[/tex]
¡Saludos!
How does genetic variation make a community more stable? Include explanations of how genetic variation is related to the struggle to survive, differential reproductive success, heredity, natural selection, and evolution
Answers
Answer:
If you have genetic variation then a community is more likely to survive because the population is more likely to have individuals who are fine if there are environmental changes. Then natural selection will happen and the species will evolve to he adapted to the new environment. If everyone in a population has the same genetics, than one thing could take them all out.
Convert 444 cal to joules
Answers
Answer: 1
Decimal Answer: 1.858
_H2+O2=_H2O
balance help please
Answers
2H2+O2=2H2O