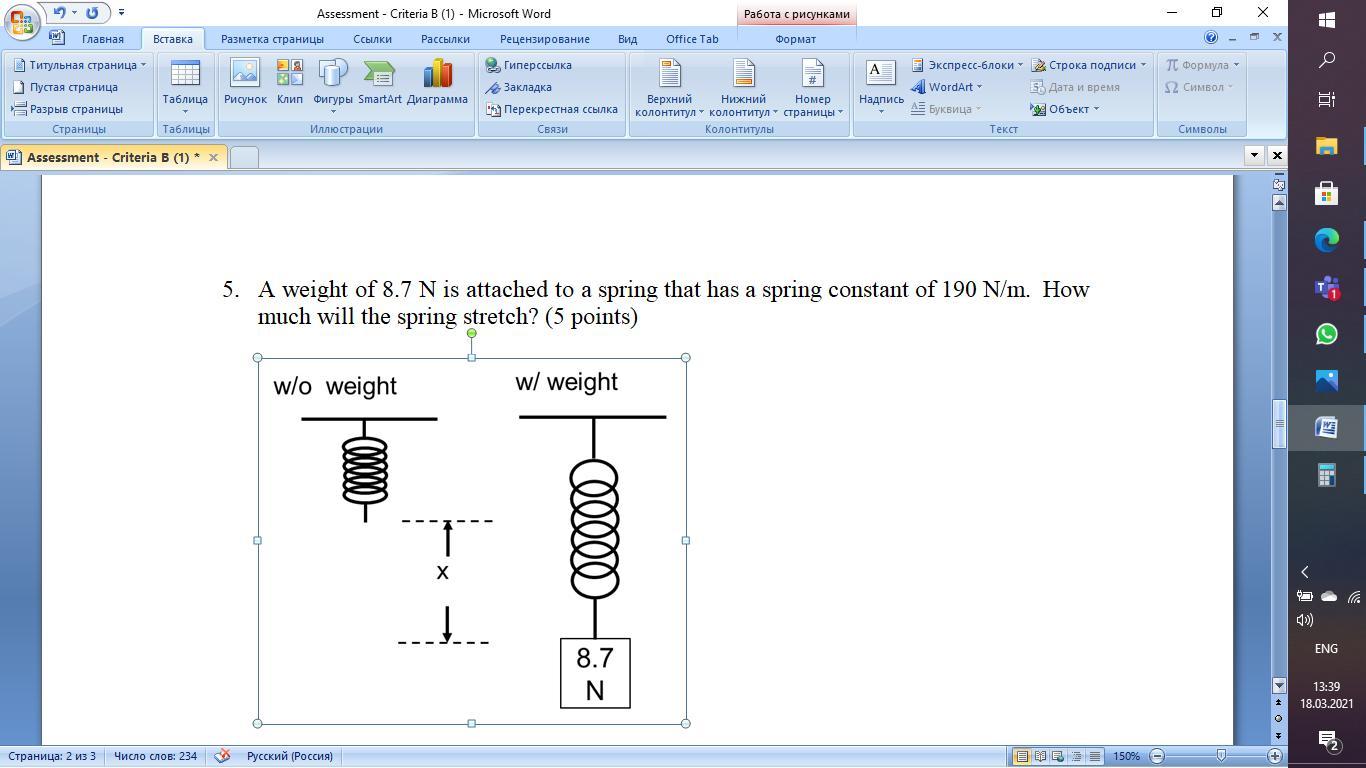
A weight of 8.7 N is attached to a spring that has a spring constant of 190 N/m. How much will the spring stretch?
Answers
Fs=8.7N
K=190N/m
Unkown:
X=?
Equation:
Fs=Kx
8.7N=(190N/m)x
X=4.6*10power of-2m
Given values are:
Force, Fs = 8.7 NSpring constant, k = 190 N/mAs we know the relation,
→ [tex]Fs = K\times x[/tex]
or,
→ [tex]x = \frac{Fs}{k}[/tex]
By putting the values, we get
[tex]= \frac{8.7}{190}[/tex]
[tex]= 4.6\times 10^{-2} \ m[/tex]
Thus the response above is right.
Learn more about spring constant here:
https://brainly.com/question/15404289
Related Questions
PLEASE HELP ASAP
During convection heat is transferred through fluids. Which statement is true about fluids?
Only gasses are fluids
Only liquids are fluids
Only solids are fluids
Both liquids and gasses are fluids
Answers
how many molecules are there in 0.45 moles of so3
Answers
Answer:
2.71
Explanation:
what physical properties does radium have
Answers
Answer:
Radium is silvery, lustrous, soft, intensely radioactive. It readily oxidizes on exposure to air, turning from almost pure white to black. Radium is luminescent, corrodes in water to form radium hydroxide. Although is the heaviest member of the alkaline-earth group it is the most volatile..
if answered correctly i will give brainlest
Answers
Answer: Earthquake location
Explanation:
Answer:
Volcano chains and arcs, and earthquake locations
Please help with this question
Answers
THE ANSWER IS c:0%
HOPE IT MAY HELPS YOUDIMENTIONAL ANALYSIS!
‼️ASAP!!! BRAINLIEST!!‼️
PLS HELP!!! SHOW ALL WORK + STEPS!! Thx!
Answers
Answer:
1037.56 mph
Explanation:
We are told the distance around the earth which is the circumference is 40075 km.
Converting to miles gives 24901.451 miles
Formula for speed = distance/time
Time for one rotation is 24 hours.
Thus, speed = 24901.451/24 = 1037.56 mph
Plz help! I will give brainliest.
Answers
Answer:
D. 0.50
Explanation:
Use avogadro number to find the whole work.
(Help would be greatly appreciated, chemistry is not my strong suit) How many moles of NaCl are present in a solution with a molarity of 8.59M and 125mL of a solution?
1. 1.07g
2. 62.7mol
3. 1.07mol
4. 62.7g
Answers
Molarity is a unit of concentration defined as the number of moles of solute (the substance being dissolved) per volume of solution (the solvent in which all the solute is dissolved). Mathematically, molarity is expressed as
[tex]M = \frac{\text{ mol solute}}{\text{ L solution}}[/tex].
In this question, we are given the molarity and the volume of a solution of NaCl. We can use this information to calculate the number of moles of NaCl present in the solution. Rearranging the equation to solve for moles of solute, we multiply the molarity by the volume of the solution (the units for volume must be in liters, so 125 mL is expressed as the equivalent 0.125 L):
[tex](8.59 \text{ M})(0.125 \text{ L}) = 1.07 \text{ moles of NaCl}.[/tex]
The question asks for the number of moles of NaCl in this solution, so number 3 would be correct.
Help me please
Answer & exp.
Answers
Explanation:
Some Rules Regarding Oxidation Numbers:
- Hydrogen has oxidation number of + 1 except in hydrides where it is -1
- Oxygen has oxidation number of -2 except in peroxides where it is -1
- Some elements have fixed oxidation numbers. E.g Halogen group elements has oxidation number of -1
- Oxidation number of a compound is the sum total of the individual elements and a neutral compound has oxidation number of 0.
A. HI
Hydrogen has oxidation of + 1
Oxidation number of I:
1 + x = 0
x = -1
B. PBr3
Br has oxidation number of - 1
Oxidation number of Pb:
x + 3 (-1) = 0
x = + 3
C. KH
Hydrogen has oxidation of + 1
Oxidation number of K:
1 + x = 0
x = -1
D. H3PO4
Hydrogen has oxidation number of + 1
Oxygen has oxidation number of -2
Oxidation number of P:
3(1) + x + 4(-2) = 0
3 + x - 8 =0
x = 5
Why is fluorine a gas at room temperature?
GIVING BRAINLIEST AND LOTS OF POINTS ‼️‼️
Answers
Answer:
In fluorine, the electrons are tightly held to the nuclei. The electrons have little chance to wander to one side of the molecule, so the London dispersion forces are relatively weak. At a low enough temperature the molecules will all be solids. At a high enough temperature they will all be gases.
Explanation:
I’ll really appreciate it if you help me out on this one .
Answers
Answer:
3) All the animals and plants in a desert
4) Species becoming extinct
what makes sherbet fizzy
Answers
Answer:
The chemical reaction between the citric acid and sodium bicarbonate
Explanation:
What do the colors of light combine to make _______________ light.
Answers
the answer is dure light I'm not sure of this answer ,but it might be to go with .
specific heat is defined as the amount of heat in what
Answers
Answer:
Specific heat is the amount of energy that must be added, in the form of heat, to one unit of mass of the substance in order to cause an increase of one unit in temperature.
Explanation:
Answer:
Specific heat is the amount of energy that must be added, in the form of heat, to one unit of mass of the substance in order to cause an increase of one unit in temperature.
Explanation:
How many grams of Sulfur are there in 2.05 x 10^25 molecules of Calcium Sulfate?
Answers
Answer:
1090 grams Sulfur (3 sig-figs)
Explanation:
Given 2.05 x 10²⁵ molecules CaSO₄ => 2.05 x 10²⁵ Sulfur atoms (subscript = 1 mole).
Converting 2.05 x 10²⁵ sulfur atoms to moles, divide by Avogadro's Number
(= 6.023 x 10²³ sulfur atoms / mole sulfur) => 2.05 x 10²⁵ sulfur atoms/6.023 x 10²³ sulfur atoms/mole sulfur atoms = 34.036 moles sulfur atoms.
Converting 34.036 moles sulfur to grams sulfur multiply by formula wt. of sulfur (=32g/mole S)
=> 34.036moles S x 32g/mole S = 1089.158 grams S ≅ 1090 g S (3 sig-figs)
How many electrons are in the nucleus of an atom with 2 protons and 3 neutrons
Answers
Answer:
there'd be 2 electrons
Explanation:
number of electrons= number of protons
Because it is the same as 2 protons
Pleade help me! (If you guys unfortunately I am going to report you) Thank you
Answers
Answer:
answer 3
Explanation:
How are chemical equations balanced?
Answers
Electrical energy exists when charged particles attract or repel each other.
PLEASE HELP
True or false
Answers
I think true..
Explanation:
im not sure.
Calculate the number of moles in 3.440 x 10^35 formula units of RbF. (Molar mass of RbF is 104.47g/mol).
Answers
Calculate the number of moles that are in 2.0 L of a 0.75 M solutions of NaCl
Answers
Answer:
1.5
Explanation:
0.75M=Moles/2L
At standard pressure, a sample of oxygen occupies 31 ml. What volume does the gas occupy when the pressure is 2 atm?
Answers
Answer:
I don't know
Explanation:
sorry I don't know
An atom of an element always contains
Answers
Answer:
An atom of an element always contain three fundamental particles called electrons(e-) , neutron ( n zero) and protons ( p+)
An atom of an element always contains a proton, electron and neutron.
What is an atom ?Every atom is made up of a nucleus and one or more electrons that are attached to the nucleus. One or more protons and a number of neutrons make up the nucleus. Only the most common type of hydrogen is neutron-free. Every solid, liquid, gas, and plasma is made up of atoms that are either neutral or ionized.
But when it comes to the word atom, we must go back to 400 B.C. Greece. And Democritus, a brilliant philosopher, proposed the Greek word atomos, which means uncuttable.
Every atom is made up of a nucleus and one or more electrons that are attached to the nucleus. One or more protons and a number of neutrons make up the nucleus.
Thus, An atom of an element always contains a proton, electron and neutron.
To learn more about an atom, follow the link;
https://brainly.com/question/29695801
#SPJ2
Explain what a convection current is and how it causes winds in the atmosphere.
Answers
What are the charges on ions of Group 1A, Group 3A (aluminum), and Group 5A?
Answers
Answer:
Group 1A: 1+
Group 3A: 3+
Group 5A: 3+ or 5+
Explanation:
Name the type of light interaction feeling hotter in a black shirt than a white shirt
1. Reflected
2. Absorbed
3. Transmittied
Answers
Please help pretty please
Answers
Answer: Its is a field generated by any two objects touching each other.
OR
Answer: Its is a field generated by any object with any mass.
Explanation: I AM SOOOO SORRY if i get it wrong i pretty sure the first one is right i mean like My first ansewr i typed in......... :/ :/
For a theoretical yield of 5.52 g and percent
yield of 51.7877%, calculate the actual vield
for a chemical reaction.
Answer in units of g.
Answers
Answer:
2.85868104g
Explanation:
Actual yield
Theoretical yield x 100 = 51.7877%
So, do the inverse and you get 2.85868104g
Which of the following processes is involved in the formation of sedimentary rocks?
O metamorphism
O cooling
O subduction
O deposition
Answers
Answer:
deposition
Explanation:
hope i can help
On the periodic table, what is a group? For the main groups, what characteristic
do the elements have in common?
Answers
Answer:
The s-, p-, and d-block elements of the periodic table are arranged into 18 numbered columns, or groups. The elements in each group have the same number of valence electrons. As a result, elements in the same group often display similar properties and reactivity.
Explanation:
On the periodic table, a group refers to a vertical column of elements that share similar chemical properties.
For the main groups, the characteristic do the elements have in common is valence electron configuration and chemical reactivity.
On the periodic table, a group refers to a vertical column of elements that share similar chemical properties. These elements are arranged in such a way that they have the same number of valence electrons, which are the electrons in the outermost energy level (shell) of their atoms. The number of valence electrons is a critical factor in determining the chemical behavior and reactivity of elements, as it governs their ability to form chemical bonds with other elements.
Main group elements, also known as representative elements, are located in the s-block and p-block of the periodic table. These elements are found in Groups 1, 2, and 13 to 18. Each main group is labeled with a number from 1 to 18.
Here are the main characteristics that elements in the same main group (vertical column) have in common:
1. Valence Electron Configuration: Elements in the same main group have identical valence electron configurations. For example, elements in Group 1 (alkali metals) all have one valence electron in their outermost energy level (ns¹), while elements in Group 18 (noble gases) have a full outermost energy level.
2. Chemical Reactivity: Due to their identical valence electron configuration, elements in the same group show similar chemical reactivity. They tend to form similar types of chemical bonds and react similarly with other elements to achieve a more stable electron configuration.
3. Periodic Trends: Main group elements within a group exhibit predictable trends in their physical and chemical properties as you move from the top to the bottom of the group. For example, the atomic radius tends to increase, ionization energy tends to decrease, and metallic character tends to increase as you go down a group.
To know more about periodic table here
https://brainly.com/question/28747247
#SPJ2