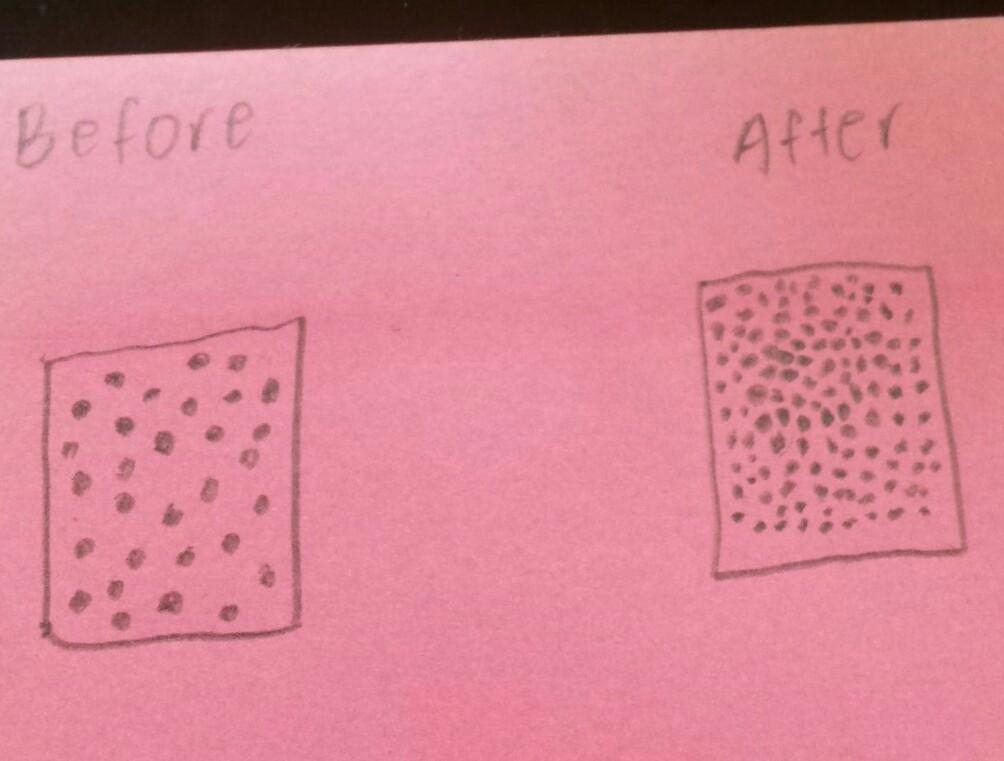
1) Draw a particle picture of liquid lemonade turning into a solid popsicle
BEFORE
AFTER
Answers
Explanation:
The particles are further apart in liquids than in solids so when the lemonade changes from a liquid to a solid, the particles would become closer.
Related Questions
How much of a 60.0 gram sample of Carbon-14 DECAYED after 17,100 if 5
its half-life= 5,700 years? *
7.50 g
52.5 g
15.0 g
30.0 g
Answers
The mass of 60 g of carbon -14 remains after 17100 years is 7.50 g. Thus, option a is correct.
What is radioactive decay?The unstable isotopes of atoms undergo radioactive decay by the emission of charged particles. The radioactive decay is a first order reaction.
The rate constant k = 1/t ln (M0/Mt)
where, M0 and Mt be the initial mass and mass after t respectively.
Given the initial mass = 60 g
half life t1/2 = 5700 years.
decay constant k = 0.693 / t1/2
= 0.693 / 5700 = 0.00012 yr⁻¹
Apply the values in the first order rate constant equation as follows:
0.00012 yr⁻¹ = 1/ (17100 yrs) ln (60/Mt)
60/Mt = 7.996
Mt = 7.50 g.
Therefore, the mass of the mass of 60 g of carbon -14 remains after 17100 years would be 7.50 g.
To find more on radioactive decay, refer here:
https://brainly.com/question/1770619
#SPJ2
The 2 in the formula AgS is a
1. coeffcient
2. subscript
3. superscript
4. binomial
Answers
Answer:
Subscript, or B
Explanation:
AgS is the equation for the chemic reaction of Silver + Sulfur, since the subscript of 2 goes by the g in the equation, this would equal subscript.
Remember subscripts are located at the BOTTOM of a coefficient and superscripts are located at the TOP of a coefficient.
Hope this supported your learning journey through and through! Don't forget to drop me a Brainliest! (No pressure, only if you want to!) (◠△◠✿) Sincerely, Kelsey from Brainly.
~ #LearnWithBrainly ~
What will the change in temperature be when 90 J are applied to 15 g of gold. (cgold = 0.126 J/g°C)
Answers
Answer:
47.6°C is the change of temperature
Explanation:
To solve this question we must use the equation of specific heat of a material:
Q = m*ΔT*S
Where Q is heat applied = 90J
m is the mass of the substance = 15g gold
ΔT is change in temperature = Our incognite
S is specific heat of the material = 0.126J/g°C for gold
Replacing:
90J = 15g*ΔT*0.126J/g°C
90J/15g*0.126J/g°C = ΔT
ΔT = 47.6°C is the change of temperature
A 125.0-g sample of a metal heated to 100.0 ∘C and placed in a calorimeter that contains 250.0 g of water. The temperature rises from 24.3 ∘C to 27.2 ∘C. What is the specific heat capacity of the metal? Ignore the calorimeter in your analysis. Group of answer choices
Answers
Answer:
0.333J/g°C is the specific heat of the metal
Explanation:
The heat that the metaal gives is equal to the heat that water is absorbing. The equation is:
S(metal)*ΔT(metal)*Mass(metal) = S(H2O)*ΔT(H2O)*Mass(H2O)
Where S is specific heat, ΔT is change in temperature and mass the mass in grams of the metal and water.
Replacing:
S(metal)*(100.0°C-27.2°C)*125.0g = 4.184J/g°C*(27.2°C-24.3°C)*250.0g
S(metal) = 4.184J/g°C*(27.2°C-24.3°C)*250.0g / (100.0°C-27.2°C)*125.0g
S(metal) = 0.333J/g°C is the specific heat of the metal
NEED ASAP!
Describe how scientific discoveries have changed our culture, national and international legislation.
Answers
Answer:
discoveries that can change little aspects of our daily lives or finding cures for diseases that have hounded humanity, scientists have been hard at work trying to push us toward a brighter future.
Explanation:
thats what i got from my research :D
For H 3 PO 4 and H 3 BO 3 , does the subscript " of hydrogen in these two formulas seem to result in additional ions in solution as it did in Group A? Explain .
Answers
Answer:
See Explanation
Explanation:
Let us consider the ionization of these compounds;
H3PO4 ⇔3H^+ + PO4^3-
H3BO3 ⇔3H^+ + BO3^3-
The next to consider is the type of electrolyte the both solution are; the both solutions are weak electrolytes and weak electrolytes do not ionize to a large extent.
The implication of this is that, not so much number of ions is added to the solution due to the poor ionization of these weak electrolytes. Hence, in spite of the subscript of 3, the conductivity of the solution does not significantly improve for the reason stated here quite unlike when strong electrolytes are used.
Which reactant is necessary for a combustion reaction?
A. Water
O B. Heat
O c. Carbon dioxide
D. Oxygen
Answers
The reactant necessary for a combustion reaction is oxygen.
(option D).
Combustion is a chemical reaction that occurs when a substance reacts with oxygen, producing heat and light. In order for combustion to take place, three elements are needed: fuel, heat, and oxygen.
Fuel is the substance that undergoes combustion, such as gasoline, wood, or methane. Heat is the energy required to initiate and sustain the reaction. Lastly, oxygen is the reactant that combines with the fuel, allowing it to burn and release energy.
When a combustion reaction occurs, the fuel and oxygen combine to form carbon dioxide (option C) and water (option A). This process is exothermic, meaning it releases energy in the form of heat and light.
For example, when wood is burned in the presence of oxygen, it undergoes combustion. The heat from a match or a spark provides the necessary activation energy to start the reaction. Oxygen from the air combines with the carbon in the wood, producing carbon dioxide and releasing energy in the form of heat and light.
In summary, oxygen is the reactant necessary for a combustion reaction. It combines with the fuel and releases energy in the form of heat and light.
(option D).
For more such questions on combustion reaction
https://brainly.com/question/13251946
#SPJ8
Helpp me please answer fast
Answers
Answer: An element is a substance only containing atoms that have the same number of protons in the chemical nuclei
Explanation:
Calculate the hydrogen ion concentration of a Nitric acid solution with a pH of 2.11.
[H] = _____ (round to 2 decimal places)
Answers
Answer:
0.01 MExplanation:
The hydrogen ion concentration can be found by finding the antilog of the pH
[tex]pH = - log [ {H}^{+} ][/tex]
We have
[tex]2.11 = - log({H}^{+}) \\ find \: \: antilog \: of \: both \: \: sides \\ \\ |{H}^{+}| = {10 }^{ - 2.11} \\ = 0.00776...[/tex]
We have the final answer as
0.01 M to 2 decimal places
Hope this helps you
Which expression represents the pH of a solution?
Answers
Answer:
pH = 14 - pOH
pH = -log[H+]
pH = pKa + log [A-]/[HA]
Explanation:
The equation used depends on what is given in the equation.
The expression that represent the pH of the solution is
pH = 14 - pOH
pH = -log[H+]
pH = pKa + log [A-]/[HA]
What is the pH of the solution?It is the quantitative measure related to the acidity or the other liquid solutions. It should be range between the 1 and 10^-14. here the numbers be like from 0 to 14.
Hence, we can say that The expression that represent the pH of the solution is
pH = 14 - pOH
pH = -log[H+]
pH = pKa + log [A-]/[HA]
Learn more about pH here: https://brainly.com/question/12052751
how many ml of 10.0 m cacl2 do we need to prepare 3.00 l of a 0.500 m cacl2 solution
Answers
Answer:
150 mL
Explanation:
From the question given above, the following data were obtained:
Molarity of stock solution (M₁) = 10 M
Volume of diluted solution (V₂) = 3 L
Molarity of diluted solution (M₂) = 0.5 M
Volume of stock solution needed (V₁) =?
The volume of stock solution needed can be obtained as follow:
M₁V₁ = M₂V₂
10 × V₁ = 0.5 × 3
10 × V₁ = 1.5
Divide both side by 10
V₁ = 1.5 / 10
V₁ = 0.15 L
Finally, we shall convert 0.15 L to mL. This can be obtained as follow:
1 L = 1000 mL
Therefore,
0.15 L = 0.15 L × 1000 mL / 1 L
0.15 L = 150 mL
Therefore, the volume of the stock solution needed is 150 mL
If an 18g object occupies 2 cm3 of space, what is its density
Answers
Which reaction type? *
combination (synthesis)
combustion
decomposition
single replacement
double replacement
Answers
Mg replaces the H in HCl to become MgCl2.
What happens as liquid water boils?
A) The molecules are destroyed.
B) The mass of the molecules decreases.
C) The molecules become separated from each other.
D) The molecules break down into hydrogen and oxygen atoms.
Answers
Answer:
C) The molecules become separated from each other.
Explanation:
what is the symbol of gold
Answers
Explanation:
Tha Symbol of gold is Au.
Answer:
symbol of gold is Au
------------------------------------------------
what do electrons in the same shell have in common
Answers
Answer:
They have the same amount of energy. They are all positively charged. They are all made up of atoms.
Answer:
they have the same quantity of energy...
At STP what is the density of a gas that has a gram molecular mass of 32 grams?
Answers
Answer:1.4 g/L
Explanation:
One mole of very substance at STP contains a volume of 22.4 liters. Density can be found by dividing the mass by volume . The density of the gas with a molecular mass of 32 grams is 1.42 g/l.
What is density?Density of a substance is the measure of its mass per volume. It represents how much denser it is in a given volume. Density depends on the molecular weight, bond type, volume, temperature and pressure.
STP is defined as the condition of standard temperature and pressure. 293 K and 1 atm pressure are considered to be the standard conditions. At STP, the volume of one mole of every substances contains 22.41 liters.
It is given that the molecular weight of the gas is 32 g/mol. Thus its density in one mole can be calculate as follows:
Density = mass/ volume.
= 32 g/ 22.4 L
= 1.42 g/L.
Hence, the density of the gas at STP with molecular mass of 32 g is 1.42 g/L.
To find more about density, refer the link below:
https://brainly.com/question/15164682
#SPJ2
188 grams of Ca(OH)2 contain how many moles?(with calculations)
Answers
How much energy does a copper sample absorb as heat if its specific heat is 0.384 J/g.°C, its mass is 8.00 g, and it is
heated from 10.0°C to 40.0°C?
Answers
Answer:
.0016
Explanation:
For specific heat problems you use the formula :
Q = mcΔT
They've given you the m, c and delta T, so you can plug in those values. (for the temperature change subtract 10 from 40 to see that it grew 30 degrees)
You're looking for Q so leave that variable in the equation. Then solve.
.Q = (8.00)(.384)(30)
Q = 92.16
92.16 J energy will be absorbed by a copper sample.
Given:
Specific heat capacity, C= 0.384 J/g °C
Temperature, T₁ = 10.0°C and T₂= 40.0°C
Mass, m=8.00 g
We know that,
The specific heat capacity is defined as the quantity of heat (J) absorbed per unit mass (g) of the material when its temperature increases or 1 °C, and its unit is J/g °C.
Heat energy in terms of specific heat energy can be calculated by using this formula:
Q= m. C. ΔT
∵ΔT= change in temperature,
ΔT=40.0-10.0°C=30°C
Now substituting the values in the above formula.
[tex]Q= 8.0*0.384*30=92.16J[/tex]
Hence, 92.16 J energy will be absorbed by a copper sample.
Learn more:
brainly.com/question/22991121
What is described by the frequency of a wave?
A. The speed the wave is traveling through space
B. The number of waves that pass a point in 1 second
C. The height of the wave from trough to peak
D. The distance from one peak to the next peak
Answers
Taking into account the definition of frecuency, the statement "The number of waves that pass a point in 1 second" describes the frequency of a wave.
Definition of frequencyFrequency is the number of vibrations that occur in a unit of time. Its unit is s⁻¹ or hertz (Hz).
In other words, the frequency in wave phenomena, such as sound, electromagnetic waves (such as radio or light), electrical signals or other waves, expresses the number of times the phenomenon is repeated per unit of time. For example, if a wave repeats ten times per second, it means that it has a frequency of ten cycles per second.
SummaryFinally, the statement "The number of waves that pass a point in 1 second" describes the frequency of a wave.
Learn more about frequency:
https://brainly.com/question/1247008
https://brainly.com/question/12990032
#SPJ1
what is tyndall effect
Answers
The scattering of the particles of the light in suspension and colloid is the Tyndall effect or scattering.
What are colloidal solutions?Colloidal and suspension are the types of solution that contains the mixture of the solute in the solvent. The colloidal solution includes the insoluble solute dispersed in the medium.
The light directed towards the colloidal solution gets scattered by the solute particles present in the solution and hence is used to prove true solutions apart from the colloidal and suspension.
Therefore, the Tyndall effect is the light scattering by colloidal solution.
Learn more about the Tyndall effect here:
https://brainly.com/question/14431922
#SPJ1
What is the voltage of a galvanic cell made with zinc (Zn) and aluminum (AI)? A. 0.92 V
B. -0.92 V
c. -2.44 V
D. 2.44 V
Answers
Answer:
The correct option is (a) "0.92 V".
Explanation:
The voltage of a galvanic cell made with zinc (Zn) and aluminum (AI) is given by :
Voltage =Ered- Eoxd
Where
Ered = reduction potential
Eoxd = oxidation potential
As per reduction standard potentials, the emf of Zinc is -0.76 V and the emf of Aluminium is -1.66 V. So,
V = 0.76 - (-1.66)
= 0.9 V
Hence, the correct option is (a) "0.92 V".
Una muestra de 0,830 g de MgCl2 se disuelve en 350 g de agua. Si la densidad de la disolución es 1,09 g/mL
Answers
Answer:
NMIKCORQW
J3WEFONCIONV WEJFQOMS;DX
Copper is a product of the reaction that occurs when dry ammonia is passed over a sample of heated copper(II) oxide. The equation for the reaction is given below. 2NH3 + 3CuO --> 3Cu + 3H2O + N2 Calculate the mass of copper produced if 0.12 dm^3 of nitrogen is produced at room temperature and pressure (rtp). (Relative atomic mass of Cu = 64; One mole of gas occupies 24 dm3 at rtp
Answers
Answer:
0.12 dm^3 x (1 mol/24 dm^3) = ? mols N2
mols Cu = ? mols N2 x (3 mols Cu/1 mol N2) = ?
Then g Cu = mols Cu x atomic mass Cu = ?
Please help this is due today! 5-11-2021
Observe the chemical reaction below, and identify if the equation is balanced or not. If it is not balanced, then what coefficient should you place for the reactants and products? Justify with scientific reasoning.
Mg + O2---------> MgO
Make sure to include the following details in your answer in complete sentences:
- Is the chemical reaction balanced?
- Is it following the law of conservation of mass, why is not following?
- What can you do to make sure that the equation becomes balanced and follow the law?
Answers
Answer:
- The chemical reaction is not balanced. There is two oxygens on the reactant's side while there's only one oxygen on the products side.
- I would not say it's following the law of conservation of mass as it's not a balanced equation.
- To balance this equation, you would need to add the coefficient of '2' to Magnesium (Mg) on the reactants side, and add the coefficient of '2' to the products side. This would make it so that there's 2 Mg's and 2 O's on both the reactant's side and products side.
edit: I hope this helped you in some way. ^^
How many molecules are in 10 grams of sugar?
Answers
Answer:
A sugar cube is supposed to be equivalent to a teaspoon of sugar. A teaspoon of sugar is about 4.2 g. Table sugar is composed of sucrose, which is a disaccharide of glucose and fructose, with a molar mass of 342.3 g/mol. This means a sugar cube contains (4.2 / 342.3 =) 0.0123 mol of sucrose, or (0.0123 x 6.022 x 10^23 =) 7.39 x 10^21 molecules of sucrose. As one molecule of sucrose contains one molecule of glucose and one molecule of fructose, then there about 7,400 million, million, million molecules of glucose in a sugar cube.
explanation:
HAHA d ko alam kung tama yan
KClO3 + H2SO4 ⇒ HClO4 + ClO2 + K2SO4 + H2O
is there a way to balance this?
Answers
Answer:
6KClO3 + 3H2SO4 → 2HClO4 + 4ClO2 + 3K2SO4 + 2H2O
Explanation:
It's a bit long because you will keep changing the coefficient to match both side.
A 6.23 g nugget of pure gold absorbed 282 J of heat. The initial temperature was 30.4 degrees Celsius. What was the correct final temperature of the gold nugget if the specific heat of gold is 0.129 J/gC
Answers
Answer:
Final temperature, T2 = 381.28°C
Explanation:
Given the following data;
Mass = 6.23 g
Initial temperature = 30.4°C
Heat capacity = 282 J
Specific heat capacity = 0.129 J/g°C
To find the final temperature;
Heat capacity is given by the formula;
[tex] Q = mcdt[/tex]
Where;
Q represents the heat capacity or quantity of heat.
m represents the mass of an object.
c represents the specific heat capacity of water.
dt represents the change in temperature.
Making dt the subject of formula, we have;
[tex] dt = \frac {Q}{mc} [/tex]
Substituting into the equation, we have;
[tex] dt = \frac {282}{6.23*0.129} [/tex]
[tex] dt = \frac {282}{0.8037} [/tex]
dt = 350.88°C
Now, the final temperature T2 is;
But, dt = T2 - T1
T2 = dt + T1
T2 = 350.88 + 30.4
Final temperature, T2 = 381.28°C
Question is in picture! Due at 3:45!!!
Answers
A student uses visible spectrophotometry to determine the concentration of CoCl2(aq) in a sample solution. First the student prepares a set of CoCl2(aq) solutions of known concentration. Then the student uses a spectrophotometer to determine the absorbance of each of the standard solutions at a wavelength of 510nm and constructs a standard curve. Finally, the student determines the absorbance of the sample of unknown concentration. The original solution used to make the solutions for the standard curve was prepared by dissolving 2.60g of CoCl2 (molar mass 130.g/mol) in enough water to make 100.mL of solution. What is the molar concentration of the solution
Answers
Answer:
0.200 M
Explanation:
Step 1: Given data
Mass of CoCl₂ (m): 2.60 gMolar mass of CoCl₂ (M): 130. g/molVolume of solution (V): 100. mL (0.100 L)Step 2: Calculate the moles (n) of CoCl₂ (solute)
We will use the following expression.
n = m / M
n = 2.60 g / (130. g/mol) = 0.0200 mol
Step 3: Calculate the molar concentration of the solution
Molarity is equal to the moles of solute divided by the liters of solution.
M = n/V
M = 0.0200 mol/0.100 L = 0.200 M